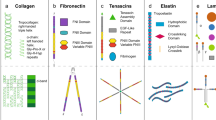
Abstract
Materials science offers a powerful tool to control mesenchymal stem cell (MSC) growth and differentiation into functional phenotypes. A complex interplay between the extracellular matrix and growth factors guides MSC phenotypes in vivo. In this Review, we discuss materials-based bioengineering approaches to direct MSC fate in vitro and in vivo, mimicking cell–matrix–growth factor crosstalk. We first scrutinize MSC–matrix interactions and how the properties of a material can be tailored to support MSC growth and differentiation in vitro, with an emphasis on MSC self-renewal mechanisms. We then highlight important growth factor signalling pathways and investigate various materials-based strategies for growth factor presentation and delivery. Integrin–growth factor crosstalk in the context of MSC engineering is introduced, and bioinspired material designs with the potential to control the MSC niche phenotype are considered. Finally, we summarize important milestones on the road to MSC engineering for regenerative medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Akhurst, R. J. & Hata, A. Targeting the TGFbeta signalling pathway in disease. Nat. Rev. Drug Discov. 11, 790–811 (2012).
Benoit, D. S., Schwartz, M. P., Durney, A. R. & Anseth, K. S. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat. Mater. 7, 816–823 (2008).
Curran, J. M., Chen, R. & Hunt, J. A. The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate. Biomaterials 27, 4783–4793 (2006).
Wen, J. H. et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 13, 979–987 (2014).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Trappmann, B. et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 11, 642–649 (2012).
Dalby, M. J. et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 6, 997–1003 (2007).
Tsimbouri, P. M. et al. Using nanotopography and metabolomics to identify biochemical effectors of multipotency. ACS Nano 6, 10239–10249 (2012).
Chen, C. S., Mrksich, M., Huang, S., Whitesides, G. & Ingber, D. E. Geometric control of cell life and death. Science 276, 1425–1428 (1997).
McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K. & Chen, C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 (2004).
Kilian, K. A., Bugarija, B., Lahn, B. T. & Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl Acad. Sci. USA 107, 4872–4877 (2010).
Celiz, A. D. et al. Discovery of a novel polymer for human pluripotent stem cell expansion and multilineage differentiation. Adv. Mater. 27, 4006–4012 (2015).
Kingham, E., White, K., Gadegaard, N., Dalby, M. J. & Oreffo, R. O. Nanotopographical cues augment mesenchymal differentiation of human embryonic stem cells. Small 9, 2140–2151 (2013).
Bible, E. et al. The support of neural stem cells transplanted into stroke-induced brain cavities by PLGA particles. Biomaterials 30, 2985–2994 (2009).
Gilbert, P. M. et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078–1081 (2010).
Guilak, F. et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5, 17–26 (2009).
Celiz, A. D. et al. Materials for stem cell factories of the future. Nat. Mater. 13, 570–579 (2014).
Discher, D. E., Mooney, D. J. & Zandstra, P. W. Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673–1677 (2009).
Kasemo, B. & Lausmaa, J. Biomaterial and implant surfaces: a surface science approach. Int. J. Oral Maxillofac. Implants 3, 247–259 (1988).
Keselowsky, B. G., Collard, D. M. & Garcia, A. J. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J. Biomed. Mater. Res. 66A, 247–259 (2003).
Ngandu Mpoyi, E. et al. Protein adsorption as a key mediator in the nanotopographical control of cell behavior. ACS Nano 10, 6638–6647 (2016).
Lee, J., Abdeen, A. A., Tang, X., Saif, T. A. & Kilian, K. A. Geometric guidance of integrin mediated traction stress during stem cell differentiation. Biomaterials 69, 174–183 (2015).
Cavalcanti-Adam, E. A., Aydin, D., Hirschfeld-Warneken, V. C. & Spatz, J. P. Cell adhesion and response to synthetic nanopatterned environments by steering receptor clustering and spatial location. HFSP J. 2, 276–285 (2008).
Cavalcanti-Adam, E. A. et al. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys. J. 92, 2964–2974 (2007).
Bershadsky, A. D., Tint, I. S., Neyfakh, A. A. Jr & Vasiliev, J. M. Focal contacts of normal and RSV-transformed quail cells. Hypothesis of the transformation-induced deficient maturation of focal contacts. Exp. Cell Res. 158, 433–444 (1985).
Biggs, M. J. et al. The use of nanoscale topography to modulate the dynamics of adhesion formation in primary osteoblasts and ERK/MAPK signalling in STRO-1+ enriched skeletal stem cells. Biomaterials 30, 5094–5103 (2009).
Kanchanawong, P. et al. Nanoscale architecture of integrin-based cell adhesions. Nature 468, 580–584 (2010).
Massia, S. P. & Hubbell, J. A. An RGD spacing of 440 nm is sufficient for integrin alpha V beta 3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J. Cell Biol. 114, 1089–1100 (1991).
McMurray, R. J. et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat. Mater. 10, 637–644 (2011).
Roberts, J. N. et al. Dynamic surfaces for the study of mesenchymal stem cell growth through adhesion regulation. ACS Nano 10, 6667–6679 (2016).
Lee, L. C. et al. Nanotopography controls cell cycle changes involved with skeletal stem cell self-renewal and multipotency. Biomaterials 116, 10–20 (2017).
Shotorbani, B. B., Alizadeh, E., Salehi, R. & Barzegar, A. Adhesion of mesenchymal stem cells to biomimetic polymers: a review. Mater. Sci. Eng. C Mater. Biol. Appl. 71, 1192–1200 (2017).
Murphy, W. L., McDevitt, T. C. & Engler, A. J. Materials as stem cell regulators. Nat. Mater. 13, 547–557 (2014).
Oria, R. et al. Force loading explains cell spatial sensing of ligands. Nature 552, 219–224 (2017).
Huang, J. et al. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 9, 1111–1116 (2009).
Huebsch, N. et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 9, 518–526 (2010).
Yang, C., Tibbitt, M. W., Basta, L. & Anseth, K. S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 13, 645–652 (2014).
Elosegui-Artola, A. et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397–1410 (2017).
Aragona, M. et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059 (2013).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Driscoll, T. P., Cosgrove, B. D., Heo, S. J., Shurden, Z. E. & Mauck, R. L. Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophys. J. 108, 2783–2793 (2015).
Cosgrove, B. D. et al. N-Cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat. Mater. 15, 1297–1306 (2016).
Bian, L., Guvendiren, M., Mauck, R. L. & Burdick, J. A. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc. Natl Acad. Sci. USA 110, 10117–10122 (2013).
Vicente, R., Noel, D., Pers, Y. M., Apparailly, F. & Jorgensen, C. Deregulation and therapeutic potential of microRNAs in arthritic diseases. Nat. Rev. Rheumatol. 12, 211–220 (2016).
Li, C. X. et al. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat. Mater. 16, 379–389 (2017).
Liu, G. et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 207, 1589–1597 (2010).
Zhu, H. et al. MicroRNA-21 in scleroderma fibrosis and its function in TGF-beta-regulated fibrosis-related genes expression. J. Clin. Immunol. 33, 1100–1109 (2013).
Liang, H. et al. A novel reciprocal loop between microRNA-21 and TGFbetaRIII is involved in cardiac fibrosis. Int. J. Biochem. Cell Biol. 44, 2152–2160 (2012).
Thum, T. et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456, 980–984 (2008).
Yao, Q. et al. Micro-RNA-21 regulates TGF-beta-induced myofibroblast differentiation by targeting PDCD4 in tumor-stroma interaction. Int. J. Cancer 128, 1783–1792 (2011).
Trohatou, O. et al. Sox2 suppression by miR-21 governs human mesenchymal stem cell properties. Stem Cells Transl Med. 3, 54–68 (2014).
Khetan, S. et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 12, 458–465 (2013).
Baker, B. M. et al. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 14, 1262–1268 (2015).
Tibbitt, M. W. & Anseth, K. S. Dynamic microenvironments: the fourth dimension. Sci. Transl Med. 4, 160–124 (2012).
Watt, F. M. & Hogan, B. L. Out of Eden: stem cells and their niches. Science 287, 1427–1430 (2000).
Kloxin, A. M., Kasko, A. M., Salinas, C. N. & Anseth, K. S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63 (2009).
DeForest, C. A. & Anseth, K. S. Photoreversible patterning of biomolecules within click-based hydrogels. Angew. Chem. Int. Ed. Engl. 51, 1816–1819 (2012).
Chaudhuri, O. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15, 326–334 (2016).
Rincon, E. et al. Mesenchymal stem cell carriers enhance antitumor efficacy of oncolytic adenoviruses in an immunocompetent mouse model. Oncotarget 11, 45415–45431 (2017).
Parker Kerrigan, B. C., Shimizu, Y., Andreeff, M. & Lang, F. F. Mesenchymal stromal cells for the delivery of oncolytic viruses in gliomas. Cytotherapy 19, 445–457 (2017).
Yoshimatsu, G. et al. The co-transplantation of bone marrow derived mesenchymal stem cells reduced inflammation in intramuscular islet transplantation. PLoS ONE 10, e0117561 (2015).
Ge, C., Xiao, G., Jiang, D. & Franceschi, R. T. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J. Cell Biol. 176, 709–718 (2007).
Ge, C. et al. Reciprocal control of osteogenic and adipogenic differentiation by ERK/MAP kinase phosphorylation of Runx2 and PPARgamma transcription factors. J. Cell. Physiol. 231, 587–596 (2016).
Yanes, O. et al. Metabolic oxidation regulates embryonic stem cell differentiation. Nat. Chem. Biol. 6, 411–417 (2010).
Seras-Franzoso, J. et al. Topographically targeted osteogenesis of mesenchymal stem cells stimulated by inclusion bodies attached to polycaprolactone surfaces. Nanomedicine 9, 207–220 (2014).
Wilson, A. et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129 (2008).
Savatier, P., Huang, S., Szekely, L., Wiman, K. G. & Samarut, J. Contrasting patterns of retinoblastoma protein expression in mouse embryonic stem cells and embryonic fibroblasts. Oncogene 9, 809–818 (1994).
White, J. et al. Developmental activation of the Rb-E2F pathway and establishment of cell cycle-regulated cyclin-dependent kinase activity during embryonic stem cell differentiation. Mol. Biol. Cell 16, 2018–2027 (2005).
Cortes, F., Debacker, C., Peault, B. & Labastie, M. C. Differential expression of KDR/VEGFR-2 and CD34 during mesoderm development of the early human embryo. Mechanisms Dev. 83, 161–164 (1999).
Salomoni, P. & Calegari, F. Cell cycle control of mammalian neural stem cells: putting a speed limit on G1. Trends Cell Biol. 20, 233–243 (2010).
Laurenti, E. et al. CDK6 levels regulate quiescence exit in human hematopoietic stem cells. Cell Stem Cell 16, 302–313 (2015).
Ogasawara, T. et al. Bone morphogenetic protein 2-induced osteoblast differentiation requires smad-mediated down-regulation of Cdk6. Mol. Cell. Biol. 24, 6560–6568 (2004).
Johnson, D. G., Schwarz, J. K., Cress, W. D. & Nevins, J. R. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature 365, 349–352 (1993).
Chong, J. L. et al. E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature 462, 930–934 (2009).
Ranga, A. et al. 3D niche microarrays for systems-level analyses of cell fate. Nat. Commun. 5, 4324 (2014).
Lutolf, M. P. & Blau, H. M. Artificial stem cell niches. Adv. Mater. 21, 3255–3268 (2009).
Mei, Y. et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat. Mater. 9, 768–778 (2010).
Curran, J. M. et al. Introducing dip pen nanolithography as a tool for controlling stem cell behaviour: unlocking the potential of the next generation of smart materials in regenerative medicine. Lab Chip 10, 1662–1670 (2010).
Das, R. K., Gocheva, V., Hammink, R., Zouani, O. F. & Rowan, A. E. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat. Mater. 15, 318–325 (2016).
Gu, H., Yue, Z., Leong, W. S., Nugraha, B. & Tan, L. P. Control of in vitro neural differentiation of mesenchymal stem cells in 3D macroporous, cellulosic hydrogels. Regen. Med. 5, 245–253 (2010).
Ehninger, A. & Trumpp, A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J. Exp. Med. 208, 421–428 (2011). This paper provides a good review of the cellular constituents of the bone marrow niche.
Bianco, P. Bone and the hematopoietic niche: a tale of two stem cells. Blood 117, 5281–5288 (2011).
Lewis, E. E. et al. A quiescent, regeneration-responsive tissue engineered mesenchymal stem cell bone marrow niche model via magnetic levitation. ACS Nano 10, 8346–8354 (2016). This paper shows a bioengineering approach to forming an in vitro MSC niche.
Pennock, R. et al. Human cell dedifferentiation in mesenchymal condensates through controlled autophagy. Sci. Rep. 5, 13113 (2015).
Lutolf, M. P., Gilbert, P. M. & Blau, H. M. Designing materials to direct stem-cell fate. Nature 462, 433–441 (2009).
Yang, C. et al. Spatially patterned matrix elasticity directs stem cell fate. Proc. Natl Acad. Sci. USA 113, E4439–E4445 (2016).
Li, L., Bennett, S. A. & Wang, L. Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell Adh. Migr. 6, 59–70 (2012).
Xu, Y. et al. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc. Natl Acad. Sci. USA 107, 8129–8134 (2010).
Rowland, T. J. et al. Roles of integrins in human induced pluripotent stem cell growth on Matrigel and vitronectin. Stem Cells Dev. 19, 1231–1240 (2010).
Nagaoka, M., Si-Tayeb, K., Akaike, T. & Duncan, S. A. Culture of human pluripotent stem cells using completely defined conditions on a recombinant E-cadherin substratum. BMC Dev. Biol. 10, 60 (2010).
Price, A. J., Huang, E. Y., Sebastiano, V. & Dunn, A. R. A semi-interpenetrating network of polyacrylamide and recombinant basement membrane allows pluripotent cell culture in a soft, ligand-rich microenvironment. Biomaterials 121, 179–192 (2017).
Chen, W. et al. Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS Nano 6, 4094–4103 (2012).
Ji, L., LaPointe, V. L., Evans, N. D. & Stevens, M. M. Changes in embryonic stem cell colony morphology and early differentiation markers driven by colloidal crystal topographical cues. Eur. Cell. Mater. 23, 135–146 (2012).
Schultz, G. S. & Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 17, 153–162 (2009).
Kleinman, H. K., Philp, D. & Hoffman, M. P. Role of the extracellular matrix in morphogenesis. Curr. Opin. Biotechnol. 14, 526–532 (2003).
Mitchell, A. C., Briquez, P. S., Hubbell, J. A. & Cochran, J. R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 30, 1–12 (2016).
Carragee, E. J., Hurwitz, E. L. & Weiner, B. K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 11, 471–491 (2011).
Yadin, D., Knaus, P. & Mueller, T. D. Structural insights into BMP receptors: specificity, activation and inhibition. Cytokine Growth Factor Rev. 27, 13–34 (2016).
Nickel, J., Sebald, W., Groppe, J. C. & Mueller, T. D. Intricacies of BMP receptor assembly. Cytokine Growth Factor Rev. 20, 367–377 (2009).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Singh, M., Berkland, C. & Detamore, M. S. Strategies and applications for incorporating physical and chemical signal gradients in tissue engineering. Tissue Eng. Part B Rev. 14, 341–366 (2008).
Liu, A. L. & Garcia, A. J. Methods for generating hydrogel particles for protein delivery. Ann. Biomed. Eng. 44, 1946–1958 (2016).
Dingal, P. C. & Discher, D. E. Combining insoluble and soluble factors to steer stem cell fate. Nat. Mater. 13, 532–537 (2014).
Rice, J. J. et al. Engineering the regenerative microenvironment with biomaterials. Adv. Healthc. Mater. 2, 57–71 (2013).
Lee, K., Silva, E. A. & Mooney, D. J. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J. R. Soc. Interface 8, 153–170 (2011).
Hynes, R. O. The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 (2009).
Migliorini, E., Valat, A., Picart, C. & Cavalcanti-Adam, E. A. Tuning cellular responses to BMP-2 with material surfaces. Cytokine Growth Factor Rev. 27, 43–54 (2016).
Kuhl, P. R. & Griffith-Cima, L. G. Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nat. Med. 2, 1022–1027 (1996).
Briquez, P. S., Hubbell, J. A. & Martino, M. M. Extracellular matrix-inspired growth factor delivery systems for skin wound healing. Adv. Wound Care 4, 479–489 (2015).
Martino, M. M., Briquez, P. S., Maruyama, K. & Hubbell, J. A. Extracellular matrix-inspired growth factor delivery systems for bone regeneration. Adv. Drug Deliv. Rev. 94, 41–52 (2015).
Briquez, P. S., Clegg, L. E., Martino, M. M., Mac Gabham, F. & Hubbell, J. A. Design principles for therapeutic angiogenic materials. Nat. Rev. Mater. 1, 15006 (2016).
Cuatrecasas, P. Interaction of insulin with the cell membrane: the primary action of insulin. Proc. Natl Acad. Sci. USA 63, 450–457 (1969).
Ito, Y., Zheng, J., Imanishi, Y., Yonezawa, K. & Kasuga, M. Protein-free cell culture on an artificial substrate with covalently immobilized insulin. Proc. Natl Acad. Sci. USA 93, 3598–3601 (1996).
Davis, M. E. et al. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc. Natl Acad. Sci. USA 103, 8155–8160 (2006).
Chen, T. T. et al. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J. Cell Biol. 188, 595–609 (2010).
Fan, V. H. et al. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells 25, 1241–1251 (2007).
Anderson, S. M., Siegman, S. N. & Segura, T. The effect of vascular endothelial growth factor (VEGF) presentation within fibrin matrices on endothelial cell branching. Biomaterials 32, 7432–7443 (2011).
Schwab, E. H. et al. Nanoscale control of surface immobilized BMP-2: toward a quantitative assessment of BMP-mediated signaling events. Nano Lett. 15, 1526–1534 (2015).
Hauff, K. et al. Matrix-immobilized BMP-2 on microcontact printed fibronectin as an in vitro tool to study BMP-mediated signaling and cell migration. Front. Bioeng. Biotechnol. 3, 62 (2015).
Azevedo, H. S. & Pashkuleva, I. Biomimetic supramolecular designs for the controlled release of growth factors in bone regeneration. Adv. Drug Deliv. Rev. 94, 63–76 (2015).
Delplace, V., Obermeyer, J. & Shoichet, M. S. Local affinity release. ACS Nano 10, 6433–6436 (2016).
Belair, D. G., Le, N. N. & Murphy, W. L. Design of growth factor sequestering biomaterials. Chem. Commun. 50, 15651–15668 (2014).
Silva, J. M., Reis, R. L. & Mano, J. F. Biomimetic extracellular environment based on natural origin polyelectrolyte multilayers. Small 12, 4308–4342 (2016).
Crouzier, T., Ren, K., Nicolas, C., Roy, C. & Picart, C. Layer-by-layer films as a biomimetic reservoir for rhBMP-2 delivery: controlled differentiation of myoblasts to osteoblasts. Small 5, 598–608 (2009).
Crouzier, T., Fourel, L., Boudou, T., Albiges-Rizo, C. & Picart, C. Presentation of BMP-2 from a soft biopolymeric film unveils its activity on cell adhesion and migration. Adv. Mater. 23, H111–H118 (2011).
Gilde, F. et al. Secondary structure of rhBMP-2 in a protective biopolymeric carrier material. Biomacromolecules 13, 3620–3626 (2012).
Roca-Cusachs, P., Iskratsch, T. & Sheetz, M. P. Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J. Cell Sci. 125, 3025–3038 (2012).
Zouani, O. F., Kalisky, J., Ibarboure, E. & Durrieu, M. C. Effect of BMP-2 from matrices of different stiffnesses for the modulation of stem cell fate. Biomaterials 34, 2157–2166 (2013).
Mammoto, A. et al. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 457, 1103–1108 (2009). This paper dissects biological mechanisms for stiffness and GF control of cell fate.
Phelps, E. A., Landazuri, N., Thule, P. M., Taylor, W. R. & Garcia, A. J. Bioartificial matrices for therapeutic vascularization. Proc. Natl Acad. Sci. USA 107, 3323–3328 (2010).
Shekaran, A. et al. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials 35, 5453–5461 (2014).
Comoglio, P. M., Boccaccio, C. & Trusolino, L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr. Opin. Cell Biol. 15, 565–571 (2003).
Wang, F. et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc. Natl Acad. Sci. USA 95, 14821–14826 (1998).
Borges, E., Jan, Y. & Ruoslahti, E. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J. Biol. Chem. 275, 39867–39873 (2000).
Schneller, M., Vuori, K. & Ruoslahti, E. Alphavbeta3 integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. EMBO J. 16, 5600–5607 (1997).
Soldi, R. et al. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 18, 882–892 (1999).
Streuli, C. H. & Akhtar, N. Signal co-operation between integrins and other receptor systems. Biochem. J. 418, 491–506 (2009).
Veevers-Lowe, J., Ball, S. G., Shuttleworth, A. & Kielty, C. M. Mesenchymal stem cell migration is regulated by fibronectin through alpha5beta1-integrin-mediated activation of PDGFR-beta and potentiation of growth factor signals. J. Cell Sci. 124, 1288–1300 (2011).
Cascone, I., Napione, L., Maniero, F., Serini, G. & Bussolino, F. Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J. Cell Biol. 170, 993–1004 (2005).
Ivaska, J. & Heino, J. Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu. Rev. Cell Dev. Biol. 27, 291–320 (2011).
Brizzi, M. F., Tarone, G. & Defilippi, P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr. Opin. Cell Biol. 24, 645–651 (2012).
Martino, M. M. & Hubbell, J. A. The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 24, 4711–4721 (2010).
Wijelath, E. S. et al. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ. Res. 99, 853–860 (2006).
Martino, M. M. et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci. Transl Med. 3, 100–189 (2011). This paper demonstrates the engineering of materials for synergistic integrin and GF signalling through the use of recombinant fragments of fibronectin.
Pankov, R. & Yamada, K. M. Fibronectin at a glance. J. Cell Sci. 115, 3861–3863 (2002).
Mao, Y. & Schwarzbauer, J. E. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 24, 389–399 (2005).
Schwarzbauer, J. E. & DeSimone, D. W. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb. Perspect. Biol. 3, a005041 (2011).
Garcia, A. J., Vega, M. D. & Boettiger, D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol. Biol. Cell 10, 785–798 (1999).
Keselowsky, B. G., Collard, D. M. & Garcia, A. J. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc. Natl Acad. Sci. USA 102, 5953–5957 (2005).
Cantini, M., Gonzalez-Garcia, C., Llopis-Hernandez, V. & Salmeron-Sanchez, M. in Proteins at Interfaces III: State of the Art (eds Horbett, T., Brash, J. L. & Norde, W. ) 471–496 (American Chemical Society, 2012).
Llopis-Hernandez, V., Cantini, M., Gonzalez-Garcia, C. & Salmeron-Sanchez, M. Material-based strategies to engineer fibronectin matrices for regenerative medicine. Int. Mater. Rev. 60, 245–263 (2015).
Salmerón-Sánchez, M. et al. Role of material-driveen fibronectin fibrillogenesis in cell differentiation. Biomaterials 32, 2099–2105 (2010).
Gugutkov, D. et al. Biological activity of the substrate-induced fibronectin network: insight into the third dimension through electrospun fibers. Langmuir 25, 10893–10900 (2009).
Llopis-Hernandez, V. et al. Material-driven fibronectin assembly for high-efficiency presentation of growth factors. Sci. Adv. 2, e1600188 (2016). This paper demonstrates a simple engineering approach for synergistic integrin and GF signalling.
Moulisova, V. et al. Engineered microenvironments for synergistic VEGF — integrin signalling during vascularization. Biomaterials 126, 61–74 (2017).
Martino, M. M. et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 343, 885–888 (2014). This paper shows that GFs have better potential when bound to the ECM than when administered as soluble factors.
Unadkat, H. V. et al. An algorithm-based topographical biomaterials library to instruct cell fate. Proc. Natl Acad. Sci. USA 108, 16565–16570 (2011).
Reimer, A. et al. Scalable topographies to support proliferation and Oct4 expression by human induced pluripotent stem cells. Sci. Rep. 6, 18948 (2016).
Gobaa, S. et al. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat. Methods 8, 949–955 (2011).
Chiappini, C. et al. Biodegradable nanoneedles for localized delivery of nanoparticles in vivo: exploring the biointerface. ACS Nano 9, 5500–5509 (2015).
Chiappini, C. et al. Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization. Nat. Mater. 14, 532–539 (2015).
Passier, R., van Laake, L. W. & Mummery, C. L. Stem-cell-based therapy and lessons from the heart. Nature 453, 322–329 (2008).
Mosiewicz, K. A. et al. In situ cell manipulation through enzymatic hydrogel photopatterning. Nat. Mater. 12, 1072–1078 (2013).
Fonseca, K. B. et al. Injectable MMP-sensitive alginate hydrogels as hMSC delivery systems. Biomacromolecules 15, 380–390 (2014).
Huebsch, N. et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 14, 1269–1277 (2015).
Crisan, M. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 (2008).
Wang, Y., Chen, X., Cao, W. & Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 15, 1009–1016 (2014).
Ayenehdeh, J. M. et al. Immunomodulatory and protective effects of adipose tissue-derived mesenchymal stem cells in an allograft islet composite transplantation for experimental autoimmune type 1 diabetes. Immunol. Lett. 188, 21–31 (2017).
Acknowledgements
The authors thank the UK Medical Research Council, the UK Engineering and Physical Sciences Research Council and the UK Biotechnology and Biological Sciences Research Council for grants MR/L022710/1, EP/P001114/1 and BB/N018419/1, respectively. The authors thank A. Rodrigo-Navarro for the design of the illustrations.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Dalby, M., García, A. & Salmeron-Sanchez, M. Receptor control in mesenchymal stem cell engineering. Nat Rev Mater 3, 17091 (2018). https://doi.org/10.1038/natrevmats.2017.91
Published:
DOI: https://doi.org/10.1038/natrevmats.2017.91