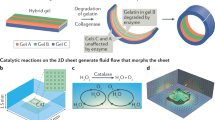
Abstract
The remarkable processes that characterize living organisms, such as motility, self-healing and reproduction, are fuelled by a continuous injection of energy at the microscale. The field of active matter focuses on understanding how the collective behaviours of internally driven components can give rise to these biological phenomena, while also striving to produce synthetic materials composed of active energy-consuming components. The synergistic approach of studying active matter in both living cells and reconstituted systems assembled from biochemical building blocks has the potential to transform our understanding of both cell biology and materials science. This methodology can provide insight into the fundamental principles that govern the dynamical behaviours of self-organizing subcellular structures, and can lead to the design of artificial materials and machines that operate away from equilibrium and can thus attain life-like properties. In this Review, we focus on active materials made of cytoskeletal components, highlighting the role of active stresses and how they drive self-organization of both cellular structures and macroscale materials, which are machines powered by nanomachines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Riskin, J. The Restless Clock: A History of the Centuries-long Argument over What Makes Living Things Tick (Univ. of Chicago Press, 2016).
Cross, M. C. & Hohenberg, P. C. Pattern formation outside of equilibrium. Rev. Mod. Phys. 65, 851–1112 (1993).
Vale, R. D. The molecular motor toolbox for intracellular transport. Cell 112, 467–480 (2003).
Jülicher, F., Ajdari, A. & Prost, J. Modeling molecular motors. Rev. Mod. Phys. 69, 1269–1281 (1997).
Nedelec, F., Surrey, T., Maggs, A. C. & Leibler, S. Self-organization of microtubules and motors. Nature 389, 305–308 (1997).
Surrey, T., Nédélec, F., Leibler, S. & Karsenti, E. Physical properties determining self-organization of motors and microtubules. Science 292, 1167–1171 (2001).
Vicsek, T., Czirók, A., Ben-Jacob, E., Cohen, I. & Shochet, O. Novel type of phase transition in a system of self-driven particles. Phys. Rev. Lett. 75, 1226–1229 (1995).
Toner, J. & Tu, Y. Flocks, herds, and schools: a quantitative theory of flocking. Phys. Rev. E 58, 4828–4858 (1998).
Simha, R. A. & Ramaswamy, S. Hydrodynamic fluctuations and instabilities in ordered suspensions of self-propelled particles. Phys. Rev. Lett. 89, 058101 (2002).
Saintillan, D. & Shelley, M. J. Active suspensions and their nonlinear models. C. R. Phys. 14, 497–517 (2013).
Ramaswamy, S. The mechanics and statistics of active matter. Annu. Rev. Condens. Matter Phys. 1, 323–345 (2010).
Toner, J., Tu, Y. & Ramaswamy, S. Hydrodynamics and phases of flocks. Ann. Phys. 318, 170–244 (2005).
Prost, J., Jülicher, F. & Joanny, J. Active gel physics. Nat. Phys. 11, 111–117 (2015).
Shelley, M. J. The dynamics of microtubule/motor-protein assemblies in biology and physics. Annu. Rev. Fluid Mechan. 48, 487–506 (2016).
Hagan, M. F. & Baskaran, A. Emergent self-organization in active materials. Curr. Opin. Cell Biol. 38, 74–80 (2016).
Marchetti, M. et al. Hydrodynamics of soft active matter. Rev. Modern Phys. 85, 1143–1189 (2013).
Fletcher, D. A. & Geissler, P. L. Active biological materials. Annu. Rev. Phys. Chem. 60, 469–486 (2009).
Schrader, F. Mitosis (Columbia Univ. Press, 1944).
Rappaport, R. Cytokinesis in Animal Cells (Cambridge Univ. Press, 1996).
Bechtel, W. Discovering Cell Mechanisms: The Creation of Modern Cell Biology (Cambridge Univ. Press, 2006).
Inoue, S., Fuseler, J., Salmon, E. D. & Ellis, G. W. Functional organization of mitotic microtubules — physical chemistry of in vivo equilibrium system. Biophys. J. 15, 725–744 (1975).
Oosawa, F. & Asakura, S. Thermodynamics of the Polymerization of Protein (Academic, 1975).
Harold, F. M. The Vital Force: A Study of Bioenergetics (W. H. Freeman, 1986).
Schrödinger, E. What is Life? With Mind and Matter and Autobiographical Sketches (Cambridge Univ. Press, 1992).
Kirschner, M. W. Implications of treadmilling for the stability and polarity of actin and tubulin polymers in vivo. J. Cell Biol. 86, 330–334 (1980).
Verde, F., Berrez, J. M., Antony, C. & Karsenti, E. Taxol-induced microtubule asters in mitotic extracts of Xenopus eggs — requirement for phosphorylated factors and cytoplasmic dynein. J. Cell Biol. 112, 1177–1187 (1991).
Mitchison, T. J. Self-organization of polymer-motor systems in the cytoskeleton. Phil. Trans. R. Soc. Lond. B Biol. Sci. 336, 99–106 (1992).
Sawin, K. E. & Scholey, J. M. Motor proteins in cell division. Trends Cell Biol. 1, 122–129 (1991).
Subramanian, R. & Kapoor, T. M. Building complexity: insights into self-organized assembly of microtubule-based architectures. Dev. Cell 23, 874–885 (2012).
Vignaud, T., Blanchoin, L. & Thery, M. Directed cytoskeleton self-organization. Trends Cell Biol. 22, 671–682 (2012).
Glick, B. S. Integrated self-organization of transitional ER and early Golgi compartments. Bioessays 36, 129–133 (2014).
Kirschner, M., Gerhart, J. & Mitchison, T. Molecular ‘vitalism’. Cell 100, 79–88 (2000).
Misteli, T. Beyond the sequence: cellular organization of genome function. Cell 128, 787–800 (2007).
Howard, J. Molecular motors: structural adaptations to cellular functions. Nature 389, 561–567 (1997).
Leibler, S. & Huse, D. A. Porters versus rowers: a unified stochastic model of motor proteins. J. Cell Biol. 121, 1357–1368 (1993).
Vale, R. D. & Milligan, R. A. The way things move: looking under the hood of molecular motor proteins. Science 288, 88–95 (2000).
Svoboda, K., Schmidt, C. F., Schnapp, B. J. & Block, S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature 365, 721–727 (1993).
Finer, J. T., Simmons, R. M. & Spudich, J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368, 113–119 (1994).
Vale, R. D. et al. Direct observation of single kinesin molecules moving along microtubules. Nature 380, 451–453 (1996).
Chen, L., Nakamura, M., Schindler, T. D., Parker, D. & Bryant, Z. Engineering controllable bidirectional molecular motors based on myosin. Nat. Nanotechnol. 7, 252–256 (2012).
Nakamura, M. et al. Remote control of myosin and kinesin motors using light-activated gearshifting. Nat. Nanotechnol. 9, 693–697 (2014).
Schindler, T. D., Chen, L., Lebel, P., Nakamura, M. & Bryant, Z. Engineering myosins for long-range transport on actin filaments. Nat. Nanotechnol. 9, 33–38 (2014).
Nédélec, F., Surrey, T. & Maggs, A. Dynamic concentration of motors in microtubule arrays. Phys. Rev. Lett. 86, 3192–3195 (2001).
Liverpool, T. B. & Marchetti, M. C. Bridging the microscopic and the hydrodynamic in active filament solutions. EPL 69, 846–852 (2005).
Chaikin, P. M. & Lubensky, T. C. Principles of Condensed Matter Physics (Cambridge Univ. Press, 2000).
Needleman, D. J. et al. Synchrotron X-ray diffraction study of microtubules buckling and bundling under osmotic stress: a probe of interprotofilament interactions. Phys. Rev. Lett. 93, 198104 (2004).
Hilitski, F. et al. Measuring cohesion between macromolecular filaments one pair at a time: depletion-induced microtubule bundling. Phys. Rev. Lett. 114, 138102 (2015).
Henkin, G., DeCamp, S. J., Chen, D. T., Sanchez, T. & Dogic, Z. Tunable dynamics of microtubule-based active isotropic gels. Phil. Trans. A. Math. Phys. Eng. Sci. 372, 20140142 (2014).
Sanchez, T., Chen, D. T., DeCamp, S. J., Heymann, M. & Dogic, Z. Spontaneous motion in hierarchically assembled active matter. Nature 491, 431–434 (2012).
Visscher, K., Schnitzer, M. J. & Block, S. M. Single kinesin molecules studied with a molecular force clamp. Nature 400, 184–189 (1999).
Szent-Györgyi, A. G. The early history of the biochemistry of muscle contraction. J. Gen. Physiol. 123, 631–641 (2004).
Szent-Györgyi, A. The contraction of myosin threads. Stud. Inst. Med. Chem. Univ. Szeged 1, 17–26 (1942).
Murrell, M., Oakes, P. W., Lenz, M. & Gardel, M. L. Forcing cells into shape: the mechanics of actomyosin contractility. Nat. Rev. Mol. Cell Biol. 16, 486–498 (2015).
Bendix, P. M. et al. A quantitative analysis of contractility in active cytoskeletal protein networks. Biophys. J. 94, 3126–3136 (2008).
Köhler, S., Schaller, V. & Bausch, A. R. Structure formation in active networks. Nat. Mater. 10, 462–468 (2011).
e Silva, M. S. et al. Active multistage coarsening of actin networks driven by myosin motors. Proc. Natl Acad. Sci. USA 108, 9408–9413 (2011).
Alvarado, J., Sheinman, M., Sharma, A., MacKintosh, F. C. & Koenderink, G. H. Molecular motors robustly drive active gels to a critically connected state. Nat. Phys. 9, 591–597 (2013).
Foster, P. J., Furthauer, S., Shelley, M. J. & Needleman, D. J. Active contraction of microtubule networks. eLife 4, e10837 (2015).
Martin, A. C., Kaschube, M. & Wieschaus, E. F. Pulsed contractions of an actin–myosin network drive apical constriction. Nature 457, 495–499 (2009).
Mayer, M., Depken, M., Bois, J. S., Jülicher, F. & Grill, S. W. Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nature 467, 617–621 (2010).
Rauzi, M., Lenne, P.-F. & Lecuit, T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468, 1110–1114 (2010).
He, L., Wang, X., Tang, H. L. & Montell, D. J. Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat. Cell Biol. 12, 1133–1142 (2010).
Shah, E. A. & Keren, K. Symmetry breaking in reconstituted actin cortices. eLife 3, e01433 (2014).
Kruse, K. & Jülicher, F. Actively contracting bundles of polar filaments. Phys. Rev. Lett. 85, 1778–1781 (2000).
Nédélec, F. & Surrey, T. Dynamics of microtubule aster formation by motor complexes. C. R. Acad. Sci. Ser. IV Phys. Astrophys. 2, 841–847 (2001).
Liverpool, T. B., Marchetti, M. C., Joanny, J.-F. & Prost, J. Mechanical response of active gels. EPL 85, 18007 (2009).
Lenz, M., Thoresen, T., Gardel, M. L. & Dinner, A. R. Contractile units in disordered actomyosin bundles arise from F-actin buckling. Phys. Rev. Lett. 108, 238107 (2012).
Murrell, M. P. & Gardel, M. L. F-Actin buckling coordinates contractility and severing in a biomimetic actomyosin cortex. Proc. Natl Acad. Sci. USA 109, 20820–20825 (2012).
Thoresen, T., Lenz, M. & Gardel, M. L. Reconstitution of contractile actomyosin bundles. Biophys. J. 100, 2698–2705 (2011).
Giomi, L., Bowick, M. J., Ma, X. & Marchetti, M. C. Defect annihilation and proliferation in active nematics. Phys. Rev. Lett. 110, 228101 (2013).
Giomi, L. Geometry and topology of turbulence in active nematics. Phys. Rev. X 5, 031003 (2015).
Thampi, S. P., Golestanian, R. & Yeomans, J. M. Velocity correlations in an active nematic. Phys. Rev. Lett. 111, 118101 (2013).
Gao, T., Blackwell, R., Glaser, M. A., Betterton, M. & Shelley, M. J. Multiscale polar theory of microtubule and motor-protein assemblies. Phys. Rev. Lett. 114, 048101 (2015).
Giomi, L., Bowick, M. J., Mishra, P., Sknepnek, R. & Marchetti, M. C. Defect dynamics in active nematics. Phil. Trans. A Math. Phys. Eng. Sci. 372, 20130365 (2014).
DeCamp, S. J., Redner, G. S., Baskaran, A., Hagan, M. F. & Dogic, Z. Orientational order of motile defects in active nematics. Nat. Mater. 14, 1110–1115 (2015).
Oza, A. U. & Dunkel, J. Antipolar ordering of topological defects in active liquid crystals. New J. Phys. 18, 093006 (2015).
Putzig, E., Redner, G. S., Baskaran, A. & Baskaran, A. Instabilities, defects, and defect ordering in an overdamped active nematic. Soft Matter 12, 3854–3859 (2016).
Doostmohammadi, A., Adamer, M. F., Thampi, S. P. & Yeomans, J. M. Stabilization of active matter by flow-vortex lattices and defect ordering. Nat. Commun. 7, 10557 (2016).
Narayan, V., Ramaswamy, S. & Menon, N. Long-lived giant number fluctuations in a swarming granular nematic. Science 317, 105–108 (2007).
Duclos, G., Garcia, S., Yevick, H. & Silberzan, P. Perfect nematic order in confined monolayers of spindle-shaped cells. Soft Matter 10, 2346–2353 (2014).
Zhou, S., Sokolov, A., Lavrentovich, O. D. & Aranson, I. S. Living liquid crystals. Proc. Natl Acad. Sci. USA 111, 1265–1270 (2014).
Bieling, P., Telley, I. A., Piehler, J. & Surrey, T. Processive kinesins require loose mechanical coupling for efficient collective motility. EMBO Rep. 9, 1121–1127 (2008).
Blackwell, R. et al. Microscopic origins of anisotropic active stress in motor-driven nematic liquid crystals. Soft Matter 12, 2676–2687 (2016).
Gao, T., Blackwell, R., Glaser, M. A., Betterton, M. & Shelley, M. J. Multiscale modeling and simulation of microtubule–motor-protein assemblies. Phys. Rev. E 92, 062709 (2015).
Guillamat, P., Ignés-Mullol, J. & Sagués, F. Control of active liquid crystals with a magnetic field. Proc. Natl Acad. Sci. USA 113, 5498–5502 (2016).
Howard, J., Hudspeth, A. & Vale, R. Movement of microtubules by single kinesin molecules. Nature 342, 154–158 (1989).
Kron, S. J. & Spudich, J. A. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc. Natl Acad. Sci. USA 83, 6272–6276 (1986).
Schaller, V., Weber, C., Semmrich, C., Frey, E. & Bausch, A. R. Polar patterns of driven filaments. Nature 467, 73–77 (2010).
Suzuki, R., Weber, C. A., Frey, E. & Bausch, A. R. Polar pattern formation in driven filament systems requires non-binary particle collisions. Nat. Phys. 11, 839–849 (2015).
Sumino, Y. et al. Large-scale vortex lattice emerging from collectively moving microtubules. Nature 483, 448–452 (2012).
Kumar, N., Soni, H., Ramaswamy, S. & Sood, A. K. Flocking at a distance in active granular matter. Nat. Commun. 5, 4688 (2014).
Bricard, A., Caussin, J.-B., Desreumaux, N., Dauchot, O. & Bartolo, D. Emergence of macroscopic directed motion in populations of motile colloids. Nature 503, 95–98 (2013).
Deseigne, J., Dauchot, O. & Chaté, H. Collective motion of vibrated polar disks. Phys. Rev. Lett. 105, 098001 (2010).
Buhl, J. et al. From disorder to order in marching locusts. Science 312, 1402–1406 (2006).
Wioland, H., Woodhouse, F. G., Dunkel, J., Kessler, J. O. & Goldstein, R. E. Confinement stabilizes a bacterial suspension into a spiral vortex. Phys. Rev. Lett. 110, 268102 (2013).
Riedel, I. H., Kruse, K. & Howard, J. A self-organized vortex array of hydrodynamically entrained sperm cells. Science 309, 300–303 (2005).
Doxzen, K. et al. Guidance of collective cell migration by substrate geometry. Integr. Biol. (Camb.) 5, 1026–1035 (2013).
Wu, K.-T. et al. Transition from turbulent to coherent flows in confined three-dimensional active fluids. Science 355, eaal1979 (2017).
Brugués, J. & Needleman, D. Physical basis of spindle self-organization. Proc. Natl Acad. Sci. USA 111, 18496–18500 (2014).
Keber, F. C. et al. Topology and dynamics of active nematic vesicles. Science 345, 1135–1139 (2014).
Nelson, D. R. Toward a tetravalent chemistry of colloids. Nano Lett. 2, 1125–1129 (2002).
Hatwalne, Y., Ramaswamy, S., Rao, M. & Simha, R. A. Rheology of active-particle suspensions. Phys. Rev. Lett. 92, 118101 (2004).
Gardel, M. L., Valentine, M. T. & Weitz, D. A. in Microscale Diagnostic Techniques 1–49 (Springer, 2005).
Lau, A. W. C., Hoffman, B. D., Davies, A., Crocker, J. C. & Lubensky, T. C. Microrheology stress fluctuations and active behavior of living cells. Phys. Rev. Lett. 91, 198101 (2003).
Mizuno, D., Tardin, C., Schmidt, C. F. & MacKintosh, F. C. Nonequilibrium mechanics of active cytoskeletal networks. Science 315, 370–373 (2007).
Chen, D. T. N. et al. Fluctuations and rheology in active bacterial suspensions. Phys. Rev. Lett. 99, 148302 (2007).
Schlosser, F., Rehfeldt, F. & Schmidt, C. F. Force fluctuations in three-dimensional suspended fibroblasts. Phil. Trans. R. Soc. Lond. B Biol. Sci. 370, 20140028 (2015).
Mizuno, D., Bacabac, R., Tardin, C., Head, D. & Schmidt, C. F. High-resolution probing of cellular force transmission. Phys. Rev. Lett. 102, 168102 (2009).
Bursac, P. et al. Cytoskeletal remodelling and slow dynamics in the living cell. Nat. Mater. 4, 557–561 (2005).
Guo, M. et al. Probing the stochastic, motor-driven properties of the cytoplasm using force spectrum microscopy. Cell 158, 822–832 (2014).
Wilhelm, C. Out-of-equilibrium microrheology inside living cells. Phys. Rev. Lett. 101, 028101 (2008).
Robert, D., Nguyen, T. H., Gallet, F. & Wilhelm, C. In vivo determination of fluctuating forces during endosome trafficking using a combination of active and passive microrheology. PLoS One 5, e10046 (2010).
MacKintosh, F. C. & Levine, A. J. Nonequilibrium mechanics and dynamics of motor-activated gels. Phys. Rev. Lett. 100, 018104 (2008).
Almonacid, M. et al. Active diffusion positions the nucleus in mouse oocytes. Nat. Cell Biol. 17, 470–479 (2015).
López, H. M., Gachelin, J., Douarche, C., Auradou, H. & Clément, E. Turning bacteria suspensions into superfluids. Phys. Rev. Lett. 115, 028301 (2015).
Naganathan, S. R., Furthauer, S., Nishikawa, M., Julicher, F. & Grill, S. W. Active torque generation by the actomyosin cell cortex drives left-right symmetry breaking. eLife 3, e04165 (2014).
Tinevez, J. Y. et al. Role of cortical tension in bleb growth. Proc. Natl Acad. Sci. USA 106, 18581–18586 (2009).
Sedzinski, J. et al. Polar actomyosin contractility destabilizes the position of the cytokinetic furrow. Nature 476, 462–466 (2011).
Turlier, H., Audoly, B., Prost, J. & Joanny, J. F. Furrow constriction in animal cell cytokinesis. Biophys. J. 106, 114–123 (2014).
Sain, A., Inamdar, M. M. & Jülicher, F. Dynamic force balances and cell shape changes during cytokinesis. Phys. Rev. Lett. 114, 048102 (2015).
Ruprecht, V. et al. Cortical contractility triggers a stochastic switch to fast amoeboid cell motility. Cell 160, 673–685 (2015).
Bergert, M. et al. Force transmission during adhesion-independent migration. Nat. Cell Biol. 17, 524–529 (2015).
Aranson, I. S. Physical Models of Cell Motility (Springer, 2016).
Löber, J., Ziebert, F. & Aranson, I. S. Modeling crawling cell movement on soft engineered substrates. Soft Matter 10, 1365–1373 (2014).
Tjhung, E., Tiribocchi, A., Marenduzzo, D. & Cates, M. E. A minimal physical model captures the shapes of crawling cells. Nat. Commun. 6, 5420 (2015).
Saha, A. et al. Determining physical properties of the cell cortex. Biophys. J. 110, 1421–1429 (2016).
Oh, D., Yu, C.-H. & Needleman, D. J. Spatial organization of the Ran pathway by microtubules in mitosis. Proc. Natl Acad. Sci. USA 113, 8729–8734 (2016).
Gowrishankar, K. et al. Active remodeling of cortical actin regulates spatiotemporal organization of cell surface molecules. Cell 149, 1353–1367 (2012).
Moseley, J. B. & Goode, B. L. The yeast actin cytoskeleton: from cellular function to biochemical mechanism. Microbiol. Mol. Biol. Rev. 70, 605–645 (2006).
Loisel, T. P., Boujemaa, R., Pantaloni, D. & Carlier, M.-F. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature 401, 613–616 (1999).
Dogterom, M. & Yurke, B. Measurement of the force–velocity relation for growing microtubules. Science 278, 856–860 (1997).
Howard, J., Grill, S. W. & Bois, J. S. Turing's next steps: the mechanochemical basis of morphogenesis. Nat. Rev. Mol. Cell Biol. 12, 392–398 (2011).
Bois, J. S., Jülicher, F. & Grill, S. W. Pattern formation in active fluids. Phys. Rev. Lett. 106, 028103 (2011).
Kumar, K. V., Bois, J. S., Jülicher, F. & Grill, S. W. Pulsatory patterns in active fluids. Phys. Rev. Lett. 112, 208101 (2014).
Bruinsma, R., Grosberg, A. Y., Rabin, Y. & Zidovska, A. Chromatin hydrodynamics. Biophys. J. 106, 1871–1881 (2014).
Zidovska, A., Weitz, D. A. & Mitchison, T. J. Micron-scale coherence in interphase chromatin dynamics. Proc. Natl Acad. Sci. USA 110, 15555–15560 (2013).
Weber, S. C., Spakowitz, A. J. & Theriot, J. A. Bacterial chromosomal loci move subdiffusively through a viscoelastic cytoplasm. Phys. Rev. Lett. 104, 238102 (2010).
Fudenberg, G. et al. Formation of chromosomal domains by loop extrusion. Cell Rep. 15, 2038–2049 (2016).
Goloborodko, A., Imakaev, M. V., Marko, J. F. & Mirny, L. Compaction and segregation of sister chromatids via active loop extrusion. eLife 5, e14864 (2016).
Naumova, N. et al. Organization of the mitotic chromosome. Science 342, 948–953 (2013).
Alipour, E. & Marko, J. F. Self-organization of domain structures by DNA-loop-extruding enzymes. Nucleic Acids Res. 40, 11202–11212 (2012).
Bertrand, O. J., Fygenson, D. K. & Saleh, O. A. Active, motor-driven mechanics in a DNA gel. Proc. Natl Acad. Sci. USA 109, 17342–17347 (2012).
Smith, K., Griffin, B., Byrd, H., MacKintosh, F. & Kilfoil, M. L. Nonthermal fluctuations of the mitotic spindle. Soft Matter 11, 4396–4401 (2015).
Dmitrieff, S., Rao, M. & Sens, P. Quantitative analysis of intra-Golgi transport shows intercisternal exchange for all cargo. Proc. Natl Acad. Sci. USA 110, 15692–15697 (2013).
Foret, L. et al. A general theoretical framework to infer endosomal network dynamics from quantitative image analysis. Curr. Biol. 22, 1381–1390 (2012).
Ramakrishnan, N., Ipsen, J. H., Rao, M. & Kumar, P. B. S. Organelle morphogenesis by active membrane remodeling. Soft Matter 11, 2387–2393 (2015).
Girard, P., Prost, J. & Bassereau, P. Passive or active fluctuations in membranes containing proteins. Phys. Rev. Lett. 94, 088102 (2005).
Faris, M. E. A. et al. Membrane tension lowering induced by protein activity. Phys. Rev. Lett. 102, 038102 (2009).
Manneville, J.-B., Bassereau, P., Levy, D. & Prost, J. Activity of transmembrane proteins induces magnification of shape fluctuations of lipid membranes. Phys. Rev. Lett. 82, 4356 (1999).
Ramaswamy, S. & Rao, M. The physics of active membranes. C. R. Acad. Sci. Ser. IV Phys. Astrophys. 2, 817–839 (2001).
He, B., Doubrovinski, K., Polyakov, O. & Wieschaus, E. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature 508, 392–396 (2014).
Farhadifar, R., Röper, J.-C., Aigouy, B., Eaton, S. & Jülicher, F. The influence of cell mechanics, cell–cell interactions, and proliferation on epithelial packing. Curr. Biol. 17, 2095–2104 (2007).
Aigouy, B. et al. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell 142, 773–786 (2010).
Hannezo, E., Prost, J. & Joanny, J.-F. Theory of epithelial sheet morphology in three dimensions. Proc. Natl Acad. Sci. USA 111, 27–32 (2014).
Zitterbart, D. P., Wienecke, B., Butler, J. P. & Fabry, B. Coordinated movements prevent jamming in an emperor penguin huddle. PLoS ONE 6, e20260 (2011).
Schwarz-Linek, J. et al. Escherichia coli as a model active colloid: a practical introduction. Colloids Surf. B 137, 2–16 (2016).
Wu, X.-L. & Libchaber, A. Particle diffusion in a quasi-two-dimensional bacterial bath. Phys. Rev. Lett. 84, 3017–3020 (2000).
Wensink, H. H. et al. Meso-scale turbulence in living fluids. Proc. Natl Acad. Sci. USA 109, 14308–14313 (2012).
Dunkel, J. et al. Fluid dynamics of bacterial turbulence. Phys. Rev. Lett. 110, 228102 (2013).
Wioland, H., Woodhouse, F. G., Dunkel, J. & Goldstein, R. E. Ferromagnetic and antiferromagnetic order in bacterial vortex lattices. Nat. Phys. 12, 341–345 (2016).
Paxton, W. F. et al. Catalytic nanomotors: autonomous movement of striped nanorods. J. Am. Chem. Soc. 126, 13424–13431 (2004).
Theurkauff, I., Cottin-Bizonne, C., Palacci, J., Ybert, C. & Bocquet, L. Dynamic clustering in active colloidal suspensions with chemical signaling. Phys. Rev. Lett. 108, 268303 (2012).
Palacci, J., Sacanna, S., Steinberg, A. P., Pine, D. J. & Chaikin, P. M. Living crystals of light-activated colloidal surfers. Science 339, 936–940 (2013).
Buttinoni, I. et al. Dynamical clustering and phase separation in suspensions of self-propelled colloidal particles. Phys. Rev. Lett. 110, 238301 (2013).
Wang, W., Chiang, T.-Y., Velegol, D. & Mallouk, T. E. Understanding the efficiency of autonomous nano- and microscale motors. J. Am. Chem. Soc. 135, 10557–10565 (2013).
Tirnauer, J. S., Salmon, E. D. & Mitchison, T. J. Microtubule plus-end dynamics in Xenopus egg extract spindles. Mol. Biol. Cell 15, 1776–1784 (2004).
Gatlin, J. C. et al. Spindle fusion requires dynein-mediated sliding of oppositely oriented microtubules. Curr. Biol. 19, 287–296 (2009).
Mitchison, T. J. et al. Roles of polymerization dynamics, opposed motors, and a tensile element in governing the length of Xenopus extract meiotic spindles. Mol. Biol. Cell 16, 3064–3076 (2005).
Schaller, V., Weber, C. A., Hammerich, B., Frey, E. & Bausch, A. R. Frozen steady states in active systems. Proc. Natl Acad. Sci. USA 108, 19183–19188 (2011).
Acknowledgements
Z.D. acknowledges primary support from Department of Energy Office of Basic Energy Science (Grant No. DE-SC0010432TDD) for supporting research on cytoskeletal active matter. Additional support from John F. Templeton Foundation (Grant Nos 57392 and NSF-MRSEC-1420382) is acknowledged. D.N. acknowledges support from the National Science Foundation (Grant Nos PHY-0847188, PHY-1305254 and DMR-0820484).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Needleman, D., Dogic, Z. Active matter at the interface between materials science and cell biology. Nat Rev Mater 2, 17048 (2017). https://doi.org/10.1038/natrevmats.2017.48
Published:
DOI: https://doi.org/10.1038/natrevmats.2017.48
This article is cited by
-
Spontaneous self-constraint in active nematic flows
Nature Physics (2024)
-
Shaping active matter from crystalline solids to active turbulence
Nature Communications (2024)
-
Autonomous waves and global motion modes in living active solids
Nature Physics (2023)
-
Inferring scale-dependent non-equilibrium activity using carbon nanotubes
Nature Nanotechnology (2023)
-
Construction of functional microtubules and artificial motile systems based on peptide design
Polymer Journal (2023)