Abstract
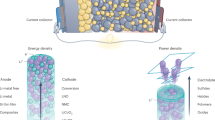
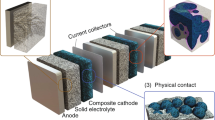
Solid-state electrolytes are attracting increasing interest for electrochemical energy storage technologies. In this Review, we provide a background overview and discuss the state of the art, ion-transport mechanisms and fundamental properties of solid-state electrolyte materials of interest for energy storage applications. We focus on recent advances in various classes of battery chemistries and systems that are enabled by solid electrolytes, including all-solid-state lithium-ion batteries and emerging solid-electrolyte lithium batteries that feature cathodes with liquid or gaseous active materials (for example, lithium–air, lithium–sulfur and lithium–bromine systems). A low-cost, safe, aqueous electrochemical energy storage concept with a ‘mediator-ion’ solid electrolyte is also discussed. Advanced battery systems based on solid electrolytes would revitalize the rechargeable battery field because of their safety, excellent stability, long cycle lives and low cost. However, great effort will be needed to implement solid-electrolyte batteries as viable energy storage systems. In this context, we discuss the main issues that must be addressed, such as achieving acceptable ionic conductivity, electrochemical stability and mechanical properties of the solid electrolytes, as well as a compatible electrolyte/electrode interface.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012).
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Cabana, J., Monconduit, L., Larcher, D. & Palacin, M. R. Beyond intercalation-based Li-ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions. Adv. Mater. 22, E170–E192 (2010).
Quartarone, E. & Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: recent advances and perspectives. Chem. Soc. Rev. 40, 2525–2540 (2011).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Goodenough, J. B. & Park, K. S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013).
Bachman, J. C. et al. Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem. Rev. 116, 140–162 (2016). This paper reviews the ion-transport mechanisms and fundamental properties of solid-state electrolytes to be used in electrochemical energy storage systems.
Hu, Y. S. Batteries: getting solid. Nat. Energy 1, 16042 (2016). This paper demonstrates a solid-state battery that can deliver 70% of its maximum capacity in just one minute at room temperature.
Linford, R. G. & Hackwood, S. Physical techniques for the study of solid electrolytes. Chem. Rev. 81, 327–364 (1981).
Sakuda, A., Hayashi, A. & Tatsumisago, M. Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery. Sci. Rep. 3, 02261 (2013).
Kamaya, N. et al. A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011).
Busche, M. R. et al. Dynamic formation of a solid–liquid electrolyte interphase and its consequences for hybrid-battery concepts. Nat. Chem. 8, 426–434 (2016).
Faraday, M. Experimental researches in electricity. Third series. Phil. Trans. R. Soc. Lond. 123, 23–54 (1833).
Takahashi, T. Early history of solid state ionics. Mater. Res. Soc. Symp. Proc. 135, 3–9 (1988).
Knödler, R. Thermal properties of sodium–sulphur cells. J. Appl. Electrochem. 14, 39–46 (1984).
Kummer, J. T., Arbor, A. & Weber, N. Thermo-electric generator. US patent 3,458,356 (1969).
Chandra, S., Lal, H. B. & Shahi, K. An electrochemical cell with solid, super-ionic Ag4KI5 as the electrolyte. J. Phys. D: Appl. Phys. 7, 194–198 (1974).
Yu Yao, Y. -F. & Kummer, J. T. Ion exchange properties of and rates of ionic diffusion in beta-alumina. J. Inorg. Nucl. Chem. 29, 2453–2457 (1967).
Reuter, B. & Hardel, K. Silbersulfidbromid und silbersulfidjodid. Angew. Chem. 72, 138–139 (1960).
Owens, B. Advances in Electrochemistry and Electrochemical Engineering (Wiley, 1971).
Fenton, D. E., Parker, J. M. & Wright, P. V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 14, 589 (1973).
Bones, R. J., Coetzer, J., Galloway, R. C. & Teagle, D. A. A sodium/iron(ii) chloride cell with a beta alumina electrolyte. J. Electrochem. Soc. 134, 2379–2382 (1987).
Coetzer, J. A. A new high-energy density battery system. J. Power Sources 18, 377–380 (1986).
Oshima, T., Kajita, M. & Okuno, A. Development of sodium-sulfur batteries. Int. J. Appl. Ceram. Technol. 1, 269–276 (2004).
Capasso, C. & Veneri, O. Experimental analysis of a Zebra battery based propulsion system for urban bus under dynamic conditions. Energy Procedia 61, 1138–1141 (2014).
Funke, K. Solid state ionics: from Michael Faraday to green energy—the European dimension. Sci. Technol. Adv. Mater. 14, 043502 (2013).
Knauth, P. & Tuller, H. L. Solid-state ionics: roots, status, and future prospects. J. Am. Ceram. Soc. 85, 1654–1680 (2002). This paper reviews the evolution of solid-state ionics over approximately the past 100 years.
Svensson, J. S. E. M. & Granqvist, C. G. Electrochromic coatings for “smart windows”. Sol. Energy Mater. 12, 391–402 (1985).
Li, H., Wang, Z. X., Chen, L. Q. & Huang, X. J. Research on advanced materials for Li-ion batteries. Adv. Mater. 21, 4593–4607 (2009).
Gao, J., Shi, S. Q. & Li, H. Brief overview of electrochemical potential in lithium ion batteries. Chin. Phys. B 25, 018210 (2016).
Li, W., Dahn, J. R. & Wainwright, D. S. Rechargeable lithium batteries with aqueous electrolytes. Science 264, 1115–1118 (1994).
Gray, F. M., MacCallum, J. R. & Vincent, C. A. Poly(ethylene oxide) - LiCF3SO3 - polystyrene electrolyte systems. Solid State Ionics 18–19, 282–286 (1986).
Gorecki, W. et al. NMR, DSC, and conductivity study of a poly(ethylene oxide) complex electrolyte: PEO(LiClO4)x . Solid State Ionics 18–19, 295–299 (1986).
Kelly, I. E., Owen, J. R. & Steele, B. C. H. Poly(ethylene oxide) electrolytes for operation at near room temperature. J. Power Sources 14, 13–21 (1985).
Abraham, K. M. & Alamgir, M. Li+-Conductive solid polymer electrolytes with liquid-like conductivity. J. Electrochem. Soc. 137, 1657–1658 (1990).
Wang, Z. X. et al. Investigation of the position of Li+ ions in a polyacrylonitrile-based electrolyte by Raman and infrared spectroscopy. Electrochim. Acta 41, 1443–1446 (1996).
Appetecchi, G. B., Croce, F. & Scrosati, B. Kinetics and stability of the lithium electrode in poly(methylmethacrylate)-based gel electrolytes. Electrochim. Acta 40, 991–997 (1995).
Iijima, T., Toyoguchi, Y. & Eda, N. Quasi-solid organic electrolytes gelatinized with polymethylmethacrylate and their applications for lithium batteries. Denki Kagaku 53, 619–623 (1985).
Choe, H. S., Giaccai, J., Alamgir, M. & Abraham, K. M. Preparation and characterization of poly(vinyl sulfone) based- and poly(vinylidene fluoride)-based electrolytes. Electrochim. Acta 40, 2289–2293 (1995).
Dudney, N. J., Bates, J. B., Zuhr, R. A., Luck, C. F. & Robertson, J. D. Sputtering of lithium compounds for preparation of electrolyte thin films. Solid State Ionics 53–56, 655–661 (1992).
Bates, J. B. et al. Electrical properties of amorphous lithium electrolyte thin films. Solid State Ionics 53–56, 647–654 (1992).
Inaguma, Y. et al. High ionic conductivity in lithium lanthanum titanate. Solid State Commun. 86, 689–693 (1993).
Goodenough, J. B., Hong, H. Y. -P. & Kafalas, J. A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 11, 203–220 (1976).
Subramanian, M. A., Subramanian, R. & Clearfield, A. Lithium ion conductors in the system AB(iv)2(PO4)3 (B = Ti, Zr and Hf). Solid State Ionics 18–19, 562–569 (1986).
Cussen, E. J. The structure of lithium garnets: cation disorder and clustering in a new family of fast Li+ conductors. Chem. Commun. 412–413 (2006).
Kasper, H. M. A new series of rare earth garnets Ln3+3M2Li+3O12(M = Te, W). Inorg. Chem. 8, 1000–1005 (1969).
Mazza, D. Remarks on a ternary phase in the La2O3–Me2O5–Li2O system (Me = Nb, Ta). Mater. Lett. 7, 205–207 (1988).
Kennedy, J. H., Sahami, S., Shea, S. W. & Zhang, Z. M. Preparation and conductivity measurements of SiS2–Li2S glasses doped with LiBr and LiCl. Solid State Ionics 18–19, 368–371 (1986).
Kennedy, J. H. & Yang, Y. A highly conductive Li+-glass system: (1 - x)(0.4SiS2-0.6Li2S)-xLil. J. Electrochem. Soc. 133, 2437–2438 (1986).
Li, H. Q., Wang, Y. G., Na, H. T., Liu, H. M. & Zhou, H. S. Rechargeable Ni-Li battery integrated aqueous/nonaqueous system. J. Am. Chem. Soc. 131, 15098–15101 (2009).
Lu, Y. H. & Goodenough, J. B. Rechargeable alkali-ion cathode-flow battery. J. Mater. Chem. 21, 10113–10117 (2011).
Wang, L., Wang, Y. G. & Xia, Y. Y. A high performance lithium-ion sulfur battery based on a Li2S cathode using a dual-phase electrolyte. Energy Environ. Sci. 8, 1551–1558 (2015). This paper is the first report of the feasibility of using a dual-phase electrolyte in a lithium–sulfur battery separated by a LISICON-type solid electrolyte.
Yu, X. W., Bi, Z. H., Zhao, F. & Manthiram, A. Hybrid lithium–sulfur batteries with a solid electrolyte membrane and lithium polysulfide catholyte. ACS Appl. Mater. Interfaces 7, 16625–16631 (2015).
Chang, Z. et al. Rechargeable Li//Br battery: a promising platform for post lithium ion batteries. J. Mater. Chem. A 2, 19444–19450 (2014).
Takemoto, K. & Yamada, H. Development of rechargeable lithium–bromine batteries with lithium ion conducting solid electrolyte. J. Power Sources 281, 334–340 (2015).
Kim, J. -K. et al. Rechargeable seawater battery and its electrochemical mechanism. ChemElectroChem 2, 328–332 (2014).
Chen, L., Guo, Z. Y., Xia, Y. Y. & Wang, Y. G. High-voltage aqueous battery approaching 3 V using an acidic–alkaline double electrolyte. Chem. Commun. 49, 2204–2206 (2013).
Dong, X. L., Wang, Y. G. & Xia, Y. G. Re-building Daniell cell with a Li-ion exchange film. Sci. Rep. 4, 6916 (2014).
Zhang, H. P. et al. Using Li+ as the electrochemical messenger to fabricate an aqueous rechargeable Zn–Cu battery. Chem. Commun. 51, 7294–7297 (2015).
Mehrer, H. Diffusion in Solids: Fundamentals, Methods, Materials, Diffusion-Controlled Processes (Springer, 2007).
Wu, M., Xu, B. & Ouyang, C. Physics of electron and lithium-ion transport in electrode materials for Li-ion batteries. Chin. Phys. B 25, 018206 (2015).
Park, M., Zhang, X. C., Chung, M. D., Less, G. B. & Sastry, A. M. A review of conduction phenomena in Li-ion batteries. J. Power Sources 195, 7904–7929 (2010).
Kumar, P. P. & Yashonath, S. Ionic conduction in the solid state. J. Chem. Sci. 118, 135–154 (2006). This paper provides a survey of experimental, theoretical and computational studies with the aim of understanding the high ionic conductivity in solid electrolytes.
Perram, J. (ed) The Physics of Superionic Conductors and Electrode Materials (Springer, 1983).
Hagenmuller, P. & Van Gool, V. (eds) Solid Electrolytes: General Principles, Characterization, Materials, Applications (Academic Press, 1978).
Angell, C. A. Mobile ions in amorphous solids. Annu. Rev. Phys. Chem. 43, 693–717 (1992).
Berthier, C. et al. Microscopic investigation of ionic-conductivity in alkali metal salts-poly(ethylene oxide) adducts. Solid State Ionics 11, 91–95 (1983).
Nitzan, A. & Ratner, M. A. Conduction in polymers: dynamic disorder transport. J. Phys. Chem. 98, 1765–1775 (1994). This paper discusses the ionic transportation mechanisms in polymer solid electrolytes.
Borodin, O. & Smith, G. D. Mechanism of ion transport in amorphous poly(ethylene oxide)/LiTFSI from molecular dynamics simulations. Macromolecules 39, 1620–1629 (2006).
Fergus, J. W. Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 195, 4554–4569 (2010).
Xia, W. H. et al. Ionic conductivity and air stability of Al-doped Li7La3Zr2O12 sintered in alumina and Pt crucibles. ACS Appl. Mater. Interfaces 8, 5335–5342 (2016).
Matsuyama, T. et al. Electrochemical properties of all-solid-state lithium batteries with amorphous titanium sulfide electrodes prepared by mechanical milling. J. Solid State Electr. 17, 2697–2701 (2013).
Hagman, L. O. & Kierkega, P. Crystal structure of NaMe2iv(PO4)3; Meiv = Ge, Ti, Zr. Acta Chem. Scand. 22, 1822–1826 (1968).
Thangadurai, V. & Weppner, W. Recent progress in solid oxide and lithium ion conducting electrolytes research. Ionics 12, 81–92 (2006). This paper reviews the progress in fast lithium-ion conductors (solid-oxide materials) with the emphasis on the correlation among composition, structure and electrical transport properties.
Casciola, M., Costantino, U., Merlini, L., Andersen, I. G. K. & Andersen, E. K. Preparation, structural characterization and conductivity of LiZr2(PO4)3 . Solid State Ionics 26, 229–235 (1988).
Martínez-Juárez, A., Rojo, J. M., Iglesias, J. E. & Sanz, J. Reversible monoclinic–rhombohedral transformation in LiSn2(PO4)3 with NASICON-type structure. Chem. Mater. 7, 1857–1862 (1995).
Aono, H., Sugimoto, E., Sadaoka, Y., Imanaka, N. & Adachi, G. Ionic conductivity and sinterability of lithium titanium phosphate system. Solid State Ionics 40–41, 38–42 (1990).
Morimoto, H. et al. Preparation of lithium ion conducting solid electrolyte of NASICON-type Li1 + xAlxTi2 - x(PO4)3 (x = 0.3) obtained by using the mechanochemical method and its application as surface modification materials of LiCoO2 cathode for lithium cell. J. Power Sources 240, 636–643 (2013).
Xu, X. X., Wen, Z. Y., Wu, X. W., Yang, X. L. & Gu, Z. H. Lithium ion-conducting glass–ceramics of Li1.5Al0.5Ge1.5(PO4)3–x Li2O (x = 0.0–0.20) with good electrical and electrochemical properties. J. Am. Ceram. Soc. 90, 2802–2806 (2007).
Xu, X. X., Wen, Z. Y., Yang, X. L. & Chen, L. D. Dense nanostructured solid electrolyte with high Li-ion conductivity by spark plasma sintering technique. Mater. Res. Bull. 43, 2334–2341 (2008).
Cruz, A. M., Ferreira, E. B. & Rodrigues, A. C. M. Controlled crystallization and ionic conductivity of a nanostructured LiAlGePO4 glass–ceramic. J. Non-Cryst. Solids 355, 2295–2301 (2009).
Fu, J. Fast Li+ ion conducting glass-ceramics in the system Li2O–Al2O3–GeO2–P2O5 . Solid State Ionics 104, 191–194 (1997).
Thokchom, J. S., Gupta, N. & Kumar, B. Superionic conductivity in a lithium aluminum germanium phosphate glass–ceramic. J. Electrochem. Soc. 155, A915–A920 (2008).
Thangadurai, V., Kaack, H. & Weppner, W. J. F. Novel fast lithium ion conduction in garnet-type Li5La3M2O12 (M = Nb, Ta). J. Am. Ceram. Soc. 86, 437–440 (2003).
Geiger, C. A. et al. Crystal chemistry and stability of “Li7La3Zr2O12” garnet: a fast lithium-ion conductor. Inorg. Chem. 50, 1089–1097 (2011).
Murugan, R., Ramakumar, S. & Janani, N. High conductive yttrium doped Li7La3Zr2O12 cubic lithium garnet. Electrochem. Commun. 13, 1373–1375 (2011).
Allen, J. L., Wolfenstine, J., Rangasamy, E. & Sakamoto, J. Effect of substitution (Ta, Al, Ga) on the conductivity of Li7La3Zr2O12 . J. Power Sources 206, 315–319 (2012).
Ohta, S., Kobayashi, T. & Asaoka, T. High lithium ionic conductivity in the garnet-type oxide Li7 - X La3(Zr2 - X, NbX)O12 (X = 0–2). J. Power Sources 196, 3342–3345 (2011).
Deviannapoorani, C., Dhivya, L., Ramakumar, S. & Murugan, R. Lithium ion transport properties of high conductive tellurium substituted Li7La3Zr2O12 cubic lithium garnets. J. Power Sources 240, 18–25 (2013).
Ahn, B. T. & Huggins, R. A. Phase behavior and conductivity of Li2SiS3 composition. Solid State Ionics 46, 237–242 (1991).
Kondo, S., Takada, K. & Yamamura, Y. New lithium ion conductors based on Li2S-SiS2 system. Solid State Ionics 53, 1183–1186 (1992).
Morimoto, H., Yamashita, H., Tatsumisago, M. & Minami, T. Mechanochemical synthesis of new amorphous materials of 60Li2S·40SiS2 with high lithium ion conductivity. J. Am. Ceram. Soc. 82, 1352–1354 (1999).
Kanno, R. & Maruyama, M. Lithium ionic conductor thio-LISICON: the Li2S GeS2 P2S5 system. J. Electrochem. Soc. 148, A742–A746 (2001).
Hayashi, A., Ohtomo, T., Mizuno, F., Tadanaga, K. & Tatsumisago, M. All-solid-state Li/S batteries with highly conductive glass–ceramic electrolytes. Electrochem. Commun. 5, 701–705 (2003).
Liu, Z. C. et al. Anomalous high ionic conductivity of nanoporous β-Li3PS4 . J. Am. Chem. Soc. 135, 975–978 (2013).
Mizuno, F., Hayashi, A., Tadanaga, K. & Tatsumisago, M. New, highly ion-conductive crystals precipitated from Li2S–P2S5 glasses. Adv. Mater. 17, 918–922 (2005).
Rangasamy, E. et al. An iodide-based Li7P2S8I superionic conductor. J. Am. Chem. Soc. 137, 1384–1387 (2015).
Hayashi, A., Muramatsu, H., Ohtomo, T., Hama, S. & Tatsumisago, M. Improved chemical stability and cyclability in Li2S–P2S5–P2O5–ZnO composite electrolytes for all-solid-state rechargeable lithium batteries. J. Alloys Compd. 591, 247–250 (2014).
Minami, K., Hayashi, A., Ujiie, S. & Tatsumisago, M. Electrical and electrochemical properties of glass–ceramic electrolytes in the systems Li2S–P2S5–P2S3 and Li2S–P2S5–P2O5 . Solid State Ionics 192, 122–125 (2011).
Muramatsu, H., Hayashi, A., Ohtomo, T., Hama, S. & Tatsumisago, M. Structural change of Li2S–P2S5 sulfide solid electrolytes in the atmosphere. Solid State Ionics 182, 116–119 (2011).
Alamgir, M. & Abraham, K. M. Li ion conductive electrolytes based on poly(vinyl chloride). J. Electrochem. Soc. 140, L96–L97 (1993).
Capiglia, C. et al. Structure and transport properties of polymer gel electrolytes based on PVdF-HFP and LiN(C2F5SO2)2 . Solid State Ionics 131, 291–299 (2000).
Feuillade, G. & Perche, P. Ion-conductive macromolecular gels and membranes for solid lithium cells. J. Appl. Electrochem. 5, 63–69 (1975).
Zhou, Y. F., Xie, S., Ge, X. W., Chen, C. H. & Amine, K. Preparation of rechargeable lithium batteries with poly(methyl methacrylate) based gel polymer electrolyte by in situ γ-ray irradiation-induced polymerization. J. Appl. Electrochem. 34, 1119–1125 (2004).
Appetecchi, G. B., Croce, F., Persi, L., Ronci, F. & Scrosati, B. Transport and interfacial properties of composite polymer electrolytes. Electrochim. Acta 45, 1481–1490 (2000). This paper demonstrates the advantages of the composite PEO–LiX polymer electrolytes in addressing the interfacial problems between lithium metal and the solid electrolyte.
Kumar, B. & Fellner, J. P. Polymer–ceramic composite protonic conductors. J. Power Sources 123, 132–136 (2003).
Miyake, N., Wainright, J. S. & Savinell, R. F. Evaluation of a sol-gel derived Nafion/silica hybrid membrane for proton electrolyte membrane fuel cell applications: I. Proton conductivity and water content. J. Electrochem. Soc. 148, A898–A904 (2001).
Chen-Yang, Y. W., Chen, H. C., Lin, F. J. & Chen, C. C. Polyacrylonitrile electrolytes: 1. A novel high-conductivity composite polymer electrolyte based on PAN, LiClO4 and α-Al2O3 . Solid State Ionics 150, 327–335 (2002).
Di Noto, V. & Zago, V. Inorganic-organic polymer electrolytes based on PEG400 and Al[OCH(CH3)2]3 I. Synthesis and vibrational characterizations. J. Electrochem. Soc. 151, A216–A223 (2004).
Liu, Y., Lee, J. Y. & Hong, L. In situ preparation of poly(ethylene oxide)–SiO2 composite polymer electrolytes. J. Power Sources 129, 303–311 (2004).
Magistris, A., Mustarelli, P., Quartarone, E. & Tomasi, C. Transport and thermal properties of (PEO)n–LiPF6 electrolytes for super-ambient applications. Solid State Ionics 136, 1241–1247 (2000).
Marcinek, M. et al. Ionic association in liquid (polyether–Al2O3–LiClO4) composite electrolytes. Solid State Ionics 176, 367–376 (2005).
Panero, S., Scrosati, B. & Greenbaum, S. G. Ionic conductivity and 7Li NMR study of poly(ethylene glycol) complexed with lithium salts. Electrochim. Acta 37, 1533–1538 (1992).
Borghini, M. C., Mastragostino, M., Passerini, S. & Scrosati, B. Electrochemical properties of polyethylene oxide-Li[(CF3SO2)2N]-gamma-LiAlO2 composite polymer electrolytes. J. Electrochem. Soc. 142, 2118–2121 (1995).
Golodnitsky, D. et al. Conduction mechanisms in concentrated LiI-polyethylene oxide-Al2O3-based solid electrolytes. J. Electrochem. Soc. 144, 3484–3491 (1997).
Krawiec, W. et al. Polymer nanocomposites: a new strategy for synthesizing solid electrolytes for rechargeable lithium batteries. J. Power Sources 54, 310–315 (1995).
Wang, C. S., Zhang, X. W. & Appleby, A. J. Solvent-free composite PEO-ceramic fiber/mat electrolytes for lithium secondary cells. J. Electrochem. Soc. 152, A205–A209 (2005).
Li, Q. et al. Cycling performances and interfacial properties of a Li/PEO-Li(CF3SO2)2N-ceramic filler/LiNi0.8Co0.2O2 cell. J. Power Sources 97–98, 795–797 (2001).
Kanehori, K., Ito, Y., Kirino, F., Miyauchi, K. & Kudo, T. Titanium disulfide films fabricated by plasma CVD. Solid State Ionics 18–19, 818–822 (1986).
Ohtsuka, H. & Yamaki, J. Electrical characteristics of Li2OV2O5SiO2 thin films. Solid State Ionics 35, 201–206 (1989).
Akridge, J. R. & Vourlis, H. Solid state batteries using vitreous solid electrolytes. Solid State Ionics 18–19, 1082–1087 (1986).
Akridge, J. R. & Vourlis, H. Performance of Li/TiS2 solid-state batteries using phosphorus chalcogenide network former glasses as solid electrolyte. Solid State Ionics 28–30, 841–846 (1988).
Bates, J. B. et al. Fabrication and characterization of amorphous lithium electrolyte thin-films and rechargeable thin-film batteries. J. Power Sources 43, 103–110 (1993).
Bates, J. B., Dudney, N. J., Neudecker, B., Ueda, A. & Evans, C. D. Thin-film lithium and lithium-ion batteries. Solid State Ionics 135, 33–45 (2000).
Bates, J. B. et al. Preferred orientation of polycrystalline LiCoO2 films. J. Electrochem. Soc. 147, 59–70 (2000).
Magistris, A., Chiodelli, G. & Villa, M. Lithium borophosphate vitreous electrolytes. J. Power Sources 14, 87–91 (1985).
Tealdi, C., Quartarone, E. & Mustarelli, P. in Rechargeable Batteries. Materials, Technologies and New Trends (eds Zhang, Z. & Zhang, S. S. ) 311–335 (Springer, 2015).
Yoon, Y., Park, C., Kim, J. & Shin, D. Characterization of lithium borophosphate glass thin film electrolytes deposited by RF-magnetron sputtering for micro-batteries. Solid State Ionics 225, 636–640 (2012).
Fleutot, B., Pecquenard, B., Martinez, H. & Levasseur, A. Lithium borophosphate thin film electrolyte as an alternative to LiPON for solder-reflow processed lithium-ion microbatteries. Solid State Ionics 249, 49–55 (2013).
Aaltonen, T., Alnes, M., Nilsen, O., Costelle, L. & Fjellvag, H. Lanthanum titanate and lithium lanthanum titanate thin films grown by atomic layer deposition. J. Mater. Chem. 20, 2877–2881 (2010).
Hamalainen, J. et al. Lithium phosphate thin films grown by atomic layer deposition. J. Electrochem. Soc. 159, A259–A263 (2012).
Comstock, D. J. & Elam, J. W. Mechanistic study of lithium aluminum oxide atomic layer deposition. J. Phys. Chem. C 117, 1677–1683 (2013).
Aaltonen, T., Nilsen, O., Magrasó, A. & Fjellvåg, H. Atomic layer deposition of Li2O–Al2O3 thin films. Chem. Mater. 23, 4669–4675 (2011).
Perng, Y. -C. et al. Synthesis of ion conducting LixAlySizO thin films by atomic layer deposition. J. Mater. Chem. A 2, 9566–9573 (2014).
Kozen, A. C., Pearse, A. J., Lin, C. F., Noked, M. & Rubloff, G. W. Atomic layer deposition of the solid electrolyte LiPON. Chem. Mater. 27, 5324–5331 (2015). This paper demonstrates an emerging technique (atomic layer deposition) for the fabrication of lithium phosphorus oxynitride (LiPON) thin-film solid electrolyte.
Haruyama, J., Sodeyama, K., Han, L. Y., Takada, K. & Tateyama, Y. Space–charge layer effect at interface between oxide cathode and sulfide electrolyte in all-solid-state lithium-ion battery. Chem. Mater. 26, 4248–4255 (2014).
Sakuda, A., Hayashi, A. & Tatsumisago, M. Intefacial observation between LiCoO2 electrode and Li2S–P2S5 solid electrolytes of all-solid-state lithium secondary batteries using transmission electron microscopy. Chem. Mater. 22, 949–956 (2010).
Sakuda, A. et al. All-solid-state lithium secondary batteries using Li2S–P2S5 solid electrolytes and LiFePO4 electrode particles with amorphous surface layer. Chem. Lett. 41, 260–261 (2012).
Kitaura, H., Hayashi, A., Tadanaga, K. & Tatsumisago, M. Improvement of electrochemical performance of all-solid-state lithium secondary batteries by surface modification of LiMn2O4 positive electrode. Solid State Ionics 192, 304–307 (2011).
Barghamadi, M. et al. Lithium–sulfur batteries–the solution is in the electrolyte, but is the electrolyte a solution? Energy Environ. Sci. 7, 3902–3920 (2014).
Yamaguchi, Y. et al. Ab initio simulations of Li/pyrite-MS2 (M = Fe, Ni) battery cells. J. Electrochem. Soc. 157, A630–A635 (2010).
Nagao, M. et al. In situ SEM study of a lithium deposition and dissolution mechanism in a bulk-type solid-state cell with a Li2S–P2S5 solid electrolyte. Phys. Chem. Chem. Phys. 15, 18600–18606 (2013).
Sahu, G. et al. Air-stable, high-conduction solid electrolytes of arsenic-substituted Li4SnS4 . Energy Environ. Sci. 7, 1053–1058 (2014).
Takahara, H. et al. All-solid-state lithium secondary battery using oxysulfide glass. Addition and coating of carbon to positive electrode. J. Electrochem. Soc. 151, A1539–A1544 (2004).
Jung, Y. S., Lee, K. T., Kim, J. H., Kwon, J. Y. & Oh, S. M. Thermo-electrochemical activation of an In–Cu intermetallic electrode for the anode in lithium secondary batteries. Adv. Funct. Mater. 18, 3010–3017 (2008).
Takada, K. et al. Solid-state lithium battery with graphite anode. Solid State Ionics 158, 269–274 (2003).
Takada, K. et al. Compatibility of lithium ion conductive sulfide glass with carbon-lithium electrode. J. Electrochem. Soc. 150, A274–A277 (2003).
Baba, M. et al. Fabrication and electrochemical characteristics of all-solid-state lithium-ion rechargeable batteries composed of LiMn2O4 positive and V2O5 negative electrodes. J. Power Sources 97–98, 798–800 (2001).
Ohta, N. et al. Enhancement of the high-rate capability of solid-state lithium batteries by nanoscale interfacial modification. Adv. Mater. 18, 2226–2230 (2006).
Takada, K. Progress and prospective of solid-state lithium batteries. Acta Mater. 61, 759–770 (2013).
Santosh, K. C., Longo, R. C., Xiong, K. & Cho, K. Electrode-electrolyte interface for solid state Li-ion batteries: point defects and mechanical strain. J. Electrochem. Soc. 161, F3104–F3110 (2014).
Ebner, M., Marone, F., Stampanoni, M. & Wood, V. Visualization and quantification of electrochemical and mechanical degradation in Li ion batteries. Science 342, 716–720 (2013).
Herbert, E. G., Tenhaeff, W. E., Dudney, N. J. & Pharr, G. M. Mechanical characterization of LiPON films using nanoindentation. Thin Solid Films 520, 413–418 (2011).
Luntz, A. C., Voss, J. & Reuter, K. Interfacial challenges in solid-state Li ion batteries. J. Phys. Chem. Lett. 6, 4599–4604 (2015).
Gwon, H. et al. Recent progress on flexible lithium rechargeable batteries. Energy Environ. Sci. 7, 538–551 (2014). This paper provides a review and perspective of flexible lithium-ion batteries and discusses how flexibility can be introduced into each component (especially the flexible electrolyte materials) of the lithium-ion batteries.
Qiu, W. L., Ma, X. H., Yang, Q. H., Fu, Y. B. & Zong, X. F. Novel preparation of nanocomposite polymer electrolyte and its application to lithium polymer batteries. J. Power Sources 138, 245–252 (2004).
Zhang, S. S., Ervin, M. H., Xu, K. & Jow, T. R. Microporous poly(acrylonitrile-methyl methacrylate) membrane as a separator of rechargeable lithium battery. Electrochim. Acta 49, 3339–3345 (2004).
Zhou, W. et al. Plating a dendrite-free lithium anode with a polymer/ceramic/polymer sandwich electrolyte. J. Am. Chem. Soc. 138, 9385–9388 (2016).
Li, J. C., Ma, C., Chi, M. F., Liang, C. D. & Dudney, N. J. Solid electrolyte: the key for high-voltage lithium batteries. Adv. Energy Mater. 5, 1401408 (2015).
Tealdi, C., Heath, J. & Islam, M. S. Feeling the strain: enhancing ionic transport in olivine phosphate cathodes for Li- and Na-ion batteries through strain effects. J. Mater. Chem. A 4, 6998–7004 (2016).
Brunetti, G. et al. Confirmation of the domino-cascade model by LiFePO4/FePO4 precession electron diffraction. Chem. Mater. 23, 4515–4524 (2011).
Xu, B., Qian, D. N., Wang, Z. Y. & Meng, Y. S. L. Recent progress in cathode materials research for advanced lithium ion batteries. Mater. Sci. Eng. R. 73, 51–65 (2012).
Semkow, K. W. & Sammells, A. F. A lithium oxygen secondary battery. J. Electrochem. Soc. 134, C412–C413 (1987).
Abraham, K. M. & Jiang, Z. A polymer electrolyte-based rechargeable lithium/oxygen battery. J. Electrochem. Soc. 143, 1–5 (1996).
Read, J. Characterization of the lithium/oxygen organic electrolyte battery. J. Electrochem. Soc. 149, A1190–A1195 (2002).
Kuboki, T., Okuyama, T., Ohsaki, T. & Takami, N. Lithium-air batteries using hydrophobic room temperature ionic liquid electrolyte. J. Power Sources 146, 766–769 (2005).
Ogasawara, T., Debart, A., Holzapfel, M., Novak, P. & Bruce, P. G. Rechargeable Li2O2 electrode for lithium batteries. J. Am. Chem. Soc. 128, 1390–1393 (2006).
Débart, A., Bao, J., Armstrong, G. & Bruce, P. G. An O2 cathode for rechargeable lithium batteries: the effect of a catalyst. J. Power Sources 174, 1177–1182 (2007).
Girishkumar, G., McCloskey, B., Luntz, A. C., Swanson, S. & Wilcke, W. Lithium–air battery: promise and challenges. J. Phys. Chem. Lett. 1, 2193–2203 (2010).
Zhou, H. S., Wang, Y. G., Li, H. Q. & He, P. The development of a new type of rechargeable batteries based on hybrid electrolytes. ChemSusChem 3, 1009–1019 (2010).
Lee, J. S. et al. Metal–air batteries with high energy density: Li–air versus Zn–air. Adv. Energy Mater. 1, 34–50 (2011).
Christensen, J. et al. A critical review of Li/air batteries. J. Electrochem. Soc. 159, R1–R30 (2012).
Kitaura, H. & Zhou, H. S. Electrochemical performance of solid-state lithium–air batteries using carbon nanotube catalyst in the air electrode. Adv. Energy Mater. 2, 889–894 (2012).
Zhang, T. et al. Li/polymer electrolyte/water stable lithium-conducting glass ceramics composite for lithium–air secondary batteries with an aqueous electrolyte. J. Electrochem. Soc. 155, A965–A969 (2008).
Zhang, T. et al. A novel high energy density rechargeable lithium/air battery. Chem. Commun. 46, 1661–1663 (2010).
He, P., Wang, Y. G. & Zhou, H. S. A Li-air fuel cell with recycle aqueous electrolyte for improved stability. Electrochem. Commun. 12, 1686–1689 (2010).
Li, L. J., Chai, S. H., Dai, S. & Manthiram, A. Advanced hybrid Li–air batteries with high-performance mesoporous nanocatalysts. Energy Environ. Sci. 7, 2630–2636 (2014). This paper demonstrates a solid-electrolyte lithium–air battery with the best cycling performance among the hybrid lithium–air battery studies.
Li, L. J., Fu, Y. Z. & Manthiram, A. Imidazole-buffered acidic catholytes for hybrid Li–air batteries with high practical energy density. Electrochem. Commun. 47, 67–70 (2014).
Li, L. J., Liu, C., He, G., Fan, D. L. & Manthiram, A. Hierarchical pore-in-pore and wire-in-wire catalysts for rechargeable Zn– and Li–air batteries with ultra-long cycle life and high cell efficiency. Energy Environ. Sci. 8, 3274–3282 (2015).
Li, L. J., Liu, S. Y. & Manthiram, A. Co3O4 nanocrystals coupled with O- and N-doped carbon nanoweb as a synergistic catalyst for hybrid Li–air batteries. Nano Energy 12, 852–860 (2015).
Li, L. J. & Manthiram, A. Dual-electrolyte lithium–air batteries: influence of catalyst, temperature, and solid-electrolyte conductivity on the efficiency and power density. J. Mater. Chem. A 1, 5121–5127 (2013).
Li, L. J. & Manthiram, A. Decoupled bifunctional air electrodes for high-performance hybrid lithium-air batteries. Nano Energy 9, 94–100 (2014).
Li, L. J. & Manthiram, A. O- and N-doped carbon nanowebs as metal-free catalysts for hybrid Li-air batteries. Adv. Energy Mater. 4, 1301795 (2014).
Li, L. J., Zhao, X. S., Fu, Y. Z. & Manthiram, A. Polyprotic acid catholyte for high capacity dual-electrolyte Li–air batteries. Phys. Chem. Chem. Phys. 14, 12737–12740 (2012).
Li, L. J., Zhao, X. S. & Manthiram, A. A dual-electrolyte rechargeable Li-air battery with phosphate buffer catholyte. Electrochem. Commun. 14, 78–81 (2012).
Manthiram, A. & Li, L. J. Hybrid and aqueous lithium-air batteries. Adv. Energy Mater. 5, 1401302 (2015). This paper provides an overview of recent developments in hybrid and aqueous lithium–air batteries and discusses the benefits of adopting a cell configuration that uses a lithium-ion solid electrolyte to protect the lithium-metal anode.
Wang, Y. G. & Zhou, H. S. A lithium-air battery with a potential to continuously reduce O2 from air for delivering energy. J. Power Sources 195, 358–361 (2010).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J. M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 11, 19–29 (2012).
Hasegawa, S. et al. Study on lithium/air secondary batteries—stability of NASICON-type lithium ion conducting glass–ceramics with water. J. Power Sources 189, 371–377 (2009).
Bresser, D., Passerini, S. & Scrosati, B. Recent progress and remaining challenges in sulfur-based lithium secondary batteries – a review. Chem. Commun. 49, 10545–10562 (2013).
Chen, R. J., Zhao, T. & Wu, F. From a historic review to horizons beyond: lithium–sulphur batteries run on the wheels. Chem. Commun. 51, 18–33 (2015).
Scheers, J., Fantini, S. & Johansson, P. A review of electrolytes for lithium–sulphur batteries. J. Power Sources 255, 204–218 (2014).
Zhang, Q., Cheng, X. B., Huang, J. Q., Peng, H. J. & Wei, F. Review of carbon materials for advanced lithium–sulfur batteries. New Carbon Mater. 29, 241–264 (2014).
Fang, X. & Peng, H. S. A revolution in electrodes: recent progress in rechargeable lithium–sulfur batteries. Small 11, 1488–1511 (2015).
Li, Z., Huang, Y. M., Yuan, L. X., Hao, Z. X. & Huang, Y. H. Status and prospects in sulfur–carbon composites as cathode materials for rechargeable lithium–sulfur batteries. Carbon 92, 41–63 (2015).
Bruce, P. G., Hardwick, L. J. & Abraham, K. M. Lithium-air and lithium-sulfur batteries. MRS Bull. 36, 506–512 (2011).
Nazar, L. F., Cuisinier, M. & Pang, Q. Lithium-sulfur batteries. MRS Bull. 39, 436–442 (2014).
Zhang, S. S. Liquid electrolyte lithium/sulfur battery: fundamental chemistry, problems, and solutions. J. Power Sources 231, 153–162 (2013).
Hu, J. J., Li, G. R. & Gao, X. P. Current status, problems and challenges in lithium–sulfur batteries. J. Inorg. Mater. 28, 1181–1186 (2013).
Song, J. X. et al. Strong lithium polysulfide chemisorption on electroactive sites of nitrogen-doped carbon composites for high-performance lithium–sulfur battery cathodes. Angew. Chem. Int. Ed. 54, 4325–4329 (2015).
Song, J. et al. Polysulfide rejection layer from alpha-lipoic acid for high performance lithium–sulfur battery. J. Mater. Chem. A 3, 323–330 (2015).
Huang, J. Q. et al. Ionic shield for polysulfides towards highly-stable lithium–sulfur batteries. Energy Environ. Sci. 7, 347–353 (2014).
Hart, C. J. et al. Rational design of sulphur host materials for Li–S batteries: correlating lithium polysulphide adsorptivity and self-discharge capacity loss. Chem. Commun. 51, 2308–2311 (2015).
Liang, X. et al. A highly efficient polysulfide mediator for lithium–sulfur batteries. Nat. Commun. 6, 5682 (2015).
Chung, S. H., Han, P., Singhal, R., Kalra, V. & Manthiram, A. Electrochemically stable rechargeable lithium–sulfur batteries with a microporous carbon nanofiber filter for polysulfide. Adv. Energy Mater. 5, 1500738 (2015).
Zhou, G. M., Paek, E., Hwang, G. S. & Manthiram, A. Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge. Nat. Commun. 6, 7760 (2015).
Su, Y. S., Fu, Y. Z., Cochell, T. & Manthiram, A. A strategic approach to recharging lithium-sulphur batteries for long cycle life. Nat. Commun. 4, 2985 (2013).
Chung, S. H. & Manthiram, A. A polyethylene glycol-supported microporous carbon coating as a polysulfide trap for utilizing pure sulfur cathodes in lithium–sulfur batteries. Adv. Mater. 26, 7352–7357 (2014).
Zheng, J. M. et al. Lewis acid–base interactions between polysulfides and metal organic framework in lithium sulfur batteries. Nano Lett. 14, 2345–2352 (2014).
Hassoun, J. & Scrosati, B. Moving to a solid-state configuration: a valid approach to making lithium-sulfur batteries viable for practical applications. Adv. Mater. 22, 5198–5203 (2010).
Hayashi, A., Ohtsubo, R., Ohtomo, T., Mizuno, F. & Tatsumisago, M. All-solid-state rechargeable lithium batteries with Li2S as a positive electrode material. J. Power Sources 183, 422–426 (2008).
Kobayashi, T. et al. All solid-state battery with sulfur electrode and thio-LISICON electrolyte. J. Power Sources 182, 621–625 (2008).
Nagao, M. et al. Reaction mechanism of all-solid-state lithium–sulfur battery with two-dimensional mesoporous carbon electrodes. J. Power Sources 243, 60–64 (2013).
Nagao, M. et al. All-solid-state Li–sulfur batteries with mesoporous electrode and thio-LISICON solid electrolyte. J. Power Sources 222, 237–242 (2013).
Li, N. et al. An aqueous dissolved polysulfide cathode for lithium–sulfur batteries. Energy Environ. Sci. 7, 3307–3312 (2014).
Yu, X., Bi, Z., Zhao, F. & Manthiram, A. Polysulfide-shuttle control in lithium-sulfur batteries with a chemically/electrochemically compatible NaSICON-type solid electrolyte. Adv. Energy Mater. 6, 1601392 (2016). This paper demonstrates an important approach in controlling the polysulfide-crossover problem in lithium–sulfur batteries with a chemically and electrochemically compatible NASICON-type lithium-ion solid electrolyte.
Wang, Q. S. et al. A shuttle effect free lithium sulfur battery based on a hybrid electrolyte. Phys. Chem. Chem. Phys. 16, 21225–21229 (2014).
Lühder, K., Schmidt, L., Schnittke, A. & Füllbier, H. A study on novel lithium-iodine and lithium-bromine solid electrolyte batteries. J. Power Sources 40, 257–263 (1992).
Chang, Z. et al. Rechargeable Li//Br battery: a promising platform for post lithium ion batteries. J. Mater. Chem. A 2, 19444–19450 (2014). This paper demonstrates a rechargeable lithium–bromine battery platform operated with a lithium-ion solid electrolyte, an aqueous bromine cathode and a non-aqueous lithium anode.
Bai, P., Viswanathan, V. & Bazant, M. Z. A dual-mode rechargeable lithium–bromine/oxygen fuel cell. J. Mater. Chem. A 3, 14165–14172 (2015).
Takemoto, K. & Yamada, H. Development of rechargeable lithium–bromine batteries with lithium ion conducting solid electrolyte Mater. Res. Soc. Symp. Proc. 1740, 381 (2015).
Bai, P. & Bazant, M. Z. Performance and degradation of a lithium-bromine rechargeable fuel cell using highly concentrated catholytes. Electrochim. Acta 202, 216–223 (2016).
Gong, M. & Dai, H. J. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 8, 23–39 (2015).
Linden, D. Handbook of Batteries 2nd edn (McGraw Hill, 1995).
Plust, H. G. Alkali batteries for electric vehicles —technical and economic aspects. Chem. Ing. Tech. 51, 583–593 (1979).
Licht, S., Wang, B. H. & Ghosh, S. Energetic iron(vi) chemistry: the super-iron battery. Science 285, 1039–1042 (1999).
Licht, S. & Yu, X. W. An alkaline periodate cathode and its unusual solubility behavior in KOH. Electrochem. Solid-State Lett. 10, A36–A39 (2007).
Köhler, J., Imanaka, N. & Adachi, G. Y. Multivalent cationic conduction in crystalline solids. Chem. Mater. 10, 3790–3812 (1998).
Ikeda, S., Kanbayashi, Y., Nomura, K., Kasai, A. & Ito, K. Solid electrolytes with multivalent cation conduction (2) zinc ion conduction in Zn-Zr-PO4 system. Solid State Ionics 40–41, 79–82 (1990).
Li, L. & Manthiram, A. Long-life, high-voltage acidic Zn–air batteries Adv. Energy Mater. 6, 1502054 (2015). This paper demonstrates a new approach for the development of zinc–air batteries with a mediator-ion solid electrolyte that enables an alkaline Zn/Zn(OH)42− redox reaction at the anode side, and an acidic oxygen reduction reaction and oxygen evolution reaction at the cathode side.
Acknowledgements
This work was supported by the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Science and Engineering under award number DE-SC0005397.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Manthiram, A., Yu, X. & Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat Rev Mater 2, 16103 (2017). https://doi.org/10.1038/natrevmats.2016.103
Published:
DOI: https://doi.org/10.1038/natrevmats.2016.103
This article is cited by
-
Unlocking Li superionic conductivity in face-centred cubic oxides via face-sharing configurations
Nature Materials (2024)
-
Deciphering the critical role of interstitial volume in glassy sulfide superionic conductors
Nature Communications (2024)
-
Monophase-homointerface electrodes intrinsically stabilize high-voltage all-solid-state batteries
Science China Chemistry (2024)
-
Monothetic and conductive network and mechanical stress releasing layer on micron-silicon anode enabling high-energy solid-state battery
Rare Metals (2024)
-
Lithium-induced graphene layer containing Li3P alloy phase to achieve ultra-stable electrode interface for lithium metal anode
Rare Metals (2024)