Abstract
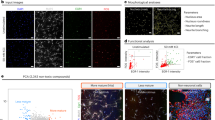
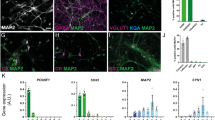
The antiepileptic drug valproic acid (VPA) has been shown to influence the neural differentiation and neurite outgrowth of neural stem cells. Sympathoadrenal progenitor cells share properties with neural stem cells and are considered a potential cell source in the treatment of neurodegenerative diseases. The present study therefore aims at modulating the neural differentiation potential of these cells by treatment with the histone deacetylase inhibitor VPA. We studied the epigenetic effects of VPA in two culture conditions: suspension conditions aimed to expand adrenomedullary sympathoadrenal progenitors within free-floating chromospheres and adherent cell cultures optimized to derive neurons. Treatment of chromospheres with VPA may launch neuronal differentiation mechanisms and improve their neurogenic potential upon transplantation. However, also transplantation of differentiated functional neurons could be beneficial. Treating chromospheres for 7 days with clinically relevant concentrations of VPA (2 mm) revealed a decrease of neural progenitor markers Nestin, Notch2 and Sox10. Furthermore, VPA initiated catecholaminergic neuronal differentiation indicated by upregulation of the neuronal marker β-III-tubulin, the dopaminergic transcription factor Pitx3 and the catecholaminergic enzymes TH and GTPCH. In adherent neural differentiation conditions, VPA treatment improved the differentiation of sympathoadrenal progenitor cells into catecholaminergic neurons with significantly elevated levels of nor- and epinephrine. In conclusion, similar to neural stem cells, VPA launches differentiation mechanisms in sympathoadrenal progenitor cells that result in increased generation of functional neurons. Thus, data from this study will be relevant to the potential use of chromaffin progenitors in transplantation therapies of neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Foti SB, Chou A, Moll AD, Roskams AJ . HDAC inhibitors dysregulate neural stem cell activity in the postnatal mouse brain. Int J Dev Neurosci 2013; 31: 434–447.
Ookubo M, Kanai H, Aoki H, Yamada N . Antidepressants and mood stabilizers effects on histone deacetylase expression in C57BL/6 mice: Brain region specific changes. J Psychiatr Res 2013; 47: 1204–1214.
Schmittwolf C, Kirchhof N, Jauch A, Durr M, Harder F, Zenke M et al. In vivo haematopoietic activity is induced in neurosphere cells by chromatin-modifying agents. EMBO J 2005; 24: 554–566.
Johannessen CU, Johannessen SI . Valproate: past, present, and future. CNS Drug Rev 2003; 9: 199–216.
Finnerup NB, Sindrup SH, Jensen TS . Chronic neuropathic pain: mechanisms, drug targets and measurement. Fundam Clin Pharm 2007; 21: 129–136.
Sykes L, Wood E, Kwan J . Antiepileptic drugs for the primary and secondary prevention of seizures after stroke. Cochrane Database Syst Rev 2014; 1: CD005398.
Laeng P, Pitts RL, Lemire AL, Drabik CE, Weiner A, Tang H et al. The mood stabilizer valproic acid stimulates GABA neurogenesis from rat forebrain stem cells. J Neurochem 2004; 91: 238–251.
De Caro V, Scaturro AL, Sutera FM, Avellone G, Schiera G, Ferrantelli E et al. N-Valproyl-l-phenylalanine as new potential antiepileptic drug: synthesis, characterization and in vitro studies on stability, toxicity and anticonvulsant efficacy. Med Chem 2014; 11: 30–40.
Whittle N, Singewald N . HDAC inhibitors as cognitive enhancers in fear, anxiety and trauma therapy: where do we stand? Biochem Soc Trans 2014; 42: 569–581.
Liu XS, Chopp M, Kassis H, Jia LF, Hozeska-Solgot A, Zhang RL et al. Valproic acid increases white matter repair and neurogenesis after stroke. Neuroscience 2012; 220: 313–321.
Go HS, Kim KC, Choi CS, Jeon SJ, Kwon KJ, Han SH et al. Prenatal exposure to valproic acid increases the neural progenitor cell pool and induces macrocephaly in rat brain via a mechanism involving the GSK-3beta/beta-catenin pathway. Neuropharmacology 2012; 63: 1028–1041.
Fukuchi M, Nii T, Ishimaru N, Minamino A, Hara D, Takasaki I et al. Valproic acid induces up- or down-regulation of gene expression responsible for the neuronal excitation and inhibition in rat cortical neurons through its epigenetic actions. Neurosci Res 2009; 65: 35–43.
Chung KF, Sicard F, Vukicevic V, Hermann A, Storch A, Huttner WB et al. Isolation of neural crest derived chromaffin progenitors from adult adrenal medulla. Stem Cells 2009; 27: 2602–2613.
Vukicevic V, Schmid J, Hermann A, Lange S, Qin N, Gebauer L et al. Differentiation of chromaffin progenitor cells to dopaminergic neurons. Cell Transplant 2012; 21: 2471–2486.
Bornstein SR, Ehrhart-Bornstein M, Androutsellis-Theotokis A, Eisenhofer G, Vukicevic V, Licinio J et al. Chromaffin cells: the peripheral brain. Mol Psychiatry 2012; 17: 354–358.
Vukicevic V, Rubin de Celis MF, Diaz-Valencia G, Bornstein SR, Ehrhart-Bornstein M . Modulation of dopaminergic neuronal differentiation from sympathoadrenal progenitors. J Mol Neurosci 2012; 48: 420–426.
Santana MM, Chung KF, Vukicevic V, Rosmaninho-Salgado J, Kanczkowski W, Cortez V et al. Isolation, characterization, and differentiation of progenitor cells from human adult adrenal medulla. Stem Cells Transl Med 2012; 1: 783–791.
Ehrhart-Bornstein M, Chung KF, Vukicevic V, Bornstein SR . Is there a role for chromaffin progenitor cells in neurodegenerative diseases? Mol Psychiatry 2009; 14: 1–4.
Vukicevic V, Jauch A, Dinger TC, Gebauer L, Hornich V, Bornstein SR et al. Genetic instability and diminished differentiation capacity in long-term cultured mouse neurosphere cells. Mech Ageing Dev 2010; 131: 124–132.
Mannering SI, Morris JS, Jensen KP, Purcell AW, Honeyman MC, van Endert PM et al. A sensitive method for detecting proliferation of rare autoantigen-specific human T cells. J Immunol Methods 2003; 283: 173–183.
Banks HT, Sutton KL, Thompson WC, Bocharov G, Roose D, Schenkel T et al. Estimation of cell proliferation dynamics using CFSE data. B Math Biol 2011; 73: 116–150.
Eisenhofer G, Goldstein DS, Stull R, Keiser HR, Sunderland T, Murphy DL et al. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem 1986; 32: 2030–2033.
Tchorz JS, Tome M, Cloetta D, Sivasankaran B, Grzmil M, Huber RM et al. Constitutive Notch2 signaling in neural stem cells promotes tumorigenic features and astroglial lineage entry. Cell Death Dis 2012; 3: e325.
Easter SS Jr, Ross LS . Frankfurter A. Initial tract formation in the mouse brain. J Neurosci 1993; 13: 285–299.
Kidd SK, Schneider JS . Protective effects of valproic acid on the nigrostriatal dopamine system in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Neuroscience 2011; 194: 189–194.
Chen S, Wu H, Klebe D, Hong Y, Zhang J . Valproic acid: a new candidate of therapeutic application for the acute central nervous system injuries. Neurochem Res 2014; 39: 1621–1633.
Yamauchi J, Torii T, Kusakawa S, Sanbe A, Nakamura K, Takashima S et al. The mood stabilizer valproic acid improves defective neurite formation caused by Charcot-Marie-Tooth disease-associated mutant Rab7 through the JNK signaling pathway. J Neurosci Res 2010; 88: 3189–3197.
Su Z, Niu W, Liu ML, Zou Y, Zhang CL . In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun 2014; 5: 3338.
Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM . Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry 2009; 195: 194–201.
De Peri L, Crescini A, Deste G, Fusar-Poli P, Sacchetti E, Vita A . Brain structural abnormalities at the onset of schizophrenia and bipolar disorder: a meta-analysis of controlled magnetic resonance imaging studies. Curr Pharm Design 2012; 18: 486–494.
Park SW, Lee JG, Seo MK, Cho HY, Lee CH, Lee JH et al. Effects of mood-stabilizing drugs on dendritic outgrowth and synaptic protein levels in primary hippocampal neurons. Bipolar Disord, e-pub ahead of print 13 October 2014; doi:10.1111/bdi.12262.
Tsai LK, Wang Z, Munasinghe J, Leng Y, Leeds P, Chuang DM . Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke 2011; 42: 2932–2939.
Bug G, Gul H, Schwarz K, Pfeifer H, Kampfmann M, Zheng X et al. Valproic acid stimulates proliferation and self-renewal of hematopoietic stem cells. Cancer Res 2005; 65: 2537–2541.
Chaurasia P, Gajzer DC, Schaniel C, D'Souza S, Hoffman R . Epigenetic reprogramming induces the expansion of cord blood stem cells. J Clin Invest 2014; 124: 2378–2395.
Seet LF, Teng E, Lai YS, Laning J, Kraus M, Wnendt S et al. Valproic acid enhances the engraftability of human umbilical cord blood hematopoietic stem cells expanded under serum-free conditions. Eur J Haematol 2009; 82: 124–132.
Borghese L, Dolezalova D, Opitz T, Haupt S, Leinhaas A, Steinfarz B et al. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells 2010; 28: 955–964.
Louvi A, Artavanis-Tsakonas S . Notch signalling in vertebrate neural development. Nat Rev Neurosci 2006; 7: 93–102.
Wall DS, Mears AJ, McNeill B, Mazerolle C, Thurig S, Wang Y et al. Progenitor cell proliferation in the retina is dependent on Notch-independent Sonic hedgehog/Hes1 activity. J Cell Biol 2009; 184: 101–112.
Curry CL, Reed LL, Nickoloff BJ, Miele L, Foreman KE . Notch-independent regulation of Hes-1 expression by c-Jun N-terminal kinase signaling in human endothelial cells. Lab Invest 2006; 86: 842–852.
Aoyama K, Delaney C, Varnum-Finney B, Kohn AD, Moon RT, Bernstein ID . The interaction of the Wnt and Notch pathways modulates natural killer versus T-cell differentiation. Stem Cells 2007; 25: 2488–2497.
Grynfeld A, Pahlman S, Axelson H . Induced neuroblastoma cell differentiation, associated with transient HES-1 activity and reduced HASH-1 expression, is inhibited by Notch1. Int J Cancer 2000; 88: 401–410.
Axelson H . The Notch signaling cascade in neuroblastoma: role of the basic helix-loop-helix proteins HASH-1 and HES-1. Cancer Lett 2004; 204: 171–178.
Maxwell SL, Ho HY, Kuehner E, Zhao S, Li M . Pitx3 regulates tyrosine hydroxylase expression in the substantia nigra and identifies a subgroup of mesencephalic dopaminergic progenitor neurons during mouse development. Dev Biol 2005; 282: 467–479.
Maxwell SL, Li M . Midbrain dopaminergic development in vivo and in vitro from embryonic stem cells. J Anat 2005; 207: 209–218.
Konstantoulas CJ, Parmar M, Li M . FoxP1 promotes midbrain identity in embryonic stem cell-derived dopamine neurons by regulating Pitx3. J Neurochem 2010; 113: 836–847.
Nemeth AH . The genetics of primary dystonias and related disorders. Brain 2002; 125: 695–721.
Wang Z, Ferdousy F, Lawal H, Huang Z, Daigle JG, Izevbaye I et al. Catecholamines up integrates dopamine synthesis and synaptic trafficking. J Neurochem 2011; 119: 1294–1305.
D'Souza A, Onem E, Patel P, La Gamma EF, Nankova BB . Valproic acid regulates catecholaminergic pathways by concentration-dependent threshold effects on TH mRNA synthesis and degradation. Brain Res 2009; 1247: 1–10.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft DFG SFB 655 ‘From cells to tissues’ (MEB, SRB), DFG KFO 252 ‘Microenvironment of the Adrenal in Health and Disease’ (EH 161/5-1 to MEB and El 855/1-1 to GE). We thank Linda Friedrich for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Vukićević, V., Qin, N., Balyura, M. et al. Valproic acid enhances neuronal differentiation of sympathoadrenal progenitor cells. Mol Psychiatry 20, 941–950 (2015). https://doi.org/10.1038/mp.2015.3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.3
This article is cited by
-
Ascorbic acid and salvianolic acid B enhance the valproic acid and 5-azacytidinemediated cardiac differentiation of mesenchymal stem cells
Molecular Biology Reports (2023)
-
Clinical epigenetics: seizing opportunities for translation
Nature Reviews Genetics (2019)
-
Epigenetic mechanisms during ageing and neurogenesis as novel therapeutic avenues in human brain disorders
Clinical Epigenetics (2017)
-
Topiramate Improves Neuroblast Differentiation of Hippocampal Dentate Gyrus in the d-Galactose-Induced Aging Mice via Its Antioxidant Effects
Cellular and Molecular Neurobiology (2017)
-
c-Jun Amino-Terminal Kinase is Involved in Valproic Acid-Mediated Neuronal Differentiation of Mouse Embryonic NSCs and Neurite Outgrowth of NSC-Derived Neurons
Neurochemical Research (2017)