Abstract
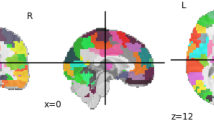
Autism is a heritable disorder, with over 250 associated genes identified to date, yet no single gene accounts for >1–2% of cases. The clinical presentation, behavioural symptoms, imaging and histopathology findings are strikingly heterogeneous. A more complete understanding of autism can be obtained by examining multiple genetic or behavioural mouse models of autism using magnetic resonance imaging (MRI)-based neuroanatomical phenotyping. Twenty-six different mouse models were examined and the consistently found abnormal brain regions across models were parieto-temporal lobe, cerebellar cortex, frontal lobe, hypothalamus and striatum. These models separated into three distinct clusters, two of which can be linked to the under and over-connectivity found in autism. These clusters also identified previously unknown connections between Nrxn1α, En2 and Fmr1; Nlgn3, BTBR and Slc6A4; and also between X monosomy and Mecp2. With no single treatment for autism found, clustering autism using neuroanatomy and identifying these strong connections may prove to be a crucial step in predicting treatment response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Abrahams BS, Geschwind DH . Connecting genes to brain in the autism spectrum disorders. Arch Neurol 2010; 67: 395–399.
Geschwind DH . Genetics of autism spectrum disorders. Trends Cogn Sci (Regul Ed) 2011; 15: 409–416.
Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A . Autism from 2 to 9 years of age. Arch Gen Psychiatry 2006; 63: 694–701.
Huerta M, Lord C . Diagnostic evaluation of autism spectrum disorders. Pediatr Clin North Am 2012; 59: 103–11–xi.
Amaral DG . The promise and the pitfalls of autism research: an introductory note for new autism researchers. Brain Res 2011; 1380: 3–9.
Canitano R . Novel treatments in autism spectrum disorders: from synaptic dysfunction to experimental therapeutics. Behav Brain Res2012; 251: 125–132.
Veenstra-VanderWeele J, Blakely RD . Networking in autism: leveraging genetic, biomarker and model system findings in the search for new treatments. Neuropsychopharmacology 2012; 37: 196–212.
Stessman HA, Bernier R, Eichler EE . A genotype-first approach to defining the subtypes of a complex disease. Cell 2014; 156: 872–877.
Hrdlicka M, Dudova I, Beranova I, Lisy J, Belsan T, Neuwirth J et al. Subtypes of autism by cluster analysis based on structural MRI data. Eur Child Adolesc Psychiatry 2005; 14: 138–144.
Banerjee-Basu S, Packer A . SFARI gene: an evolving database for the autism research community. Dis Model Mech 2010; 3: 133–135.
Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell 2009; 137: 1235–1246.
Horev G, Ellegood J, Lerch JP, Son Y-EE, Muthuswamy L, Vogel H et al. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc Natl Acad Sci USA 2011; 108: 17076–17081.
Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 2007; 318: 71–76.
Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res 2007; 176: 4–20.
International Mouse Knockout Consortium, International Mouse Knockout Consortium, Collins FS, International Mouse Knockout Consortium, Rossant J, International Mouse Knockout Consortium, Wurst W . A mouse for all reasons. Cell 2007; 128: 9–13.
Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N et al. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res 2008; 1: 147–158.
Etherton M, Földy C, Sharma M, Tabuchi K, Liu X, Shamloo M et al. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc Natl Acad Sci USA 2011; 108: 13764–13769.
Lerch JP, Gazdzinski L, Germann J, Sled JG, Henkelman RM, Nieman BJ . Wanted dead or alive? The tradeoff between in-vivo versus ex-vivo MR brain imaging in the mouse. Front Neuroinform 2012; 6: 6.
van Eede MC, Scholz J, Chakravarty MM, Henkelman RM, Lerch JP . Mapping registration sensitivity in MR mouse brain images. Neuroimage 2013; 82: 226–236.
Bock NA, Konyer NB, Henkelman RM . Multiple-mouse MRI. Magn Reson Med 2003; 49: 158–167.
Lerch JP, Sled JG, Henkelman RM . MRI phenotyping of genetically altered mice. Methods Mol Biol 2011; 711: 349–361.
Ellegood J, Babineau BA, Henkelman RM, Lerch JP, Crawley JN . Neuroanatomical analysis of the BTBR mouse model of autism using magnetic resonance imaging and diffusion tensor imaging. Neuroimage 2013; 70: 288–300.
Nieman BJ, Lerch JP, Bock NA, Chen XJ, Sled JG, Henkelman RM . Mouse behavioral mutants have neuroimaging abnormalities. Hum Brain Mapp 2007; 28: 567–575.
Lerch JP, Yiu AP, Martinez-Canabal A, Pekar T, Bohbot VD, Frankland PW et al. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. Neuroimage 2011; 54: 2086–2095.
Brodkin ES . BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res 2007; 176: 53–65.
McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN . Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav 2008; 7: 152–163.
Cahill LS, Laliberté CL, Ellegood J, Spring S, Gleave JA, Eede MCV et al. Preparation of fixed mouse brains for MRI. Neuroimage 2012; 60: 933–939.
Spring S, Lerch JP, Henkelman RM . Sexual dimorphism revealed in the structure of the mouse brain using three-dimensional magnetic resonance imaging. Neuroimage 2007; 35: 1424–1433.
Nieman BJ, Flenniken AM, Adamson SL, Henkelman RM, Sled JG . Anatomical phenotyping in the brain and skull of a mutant mouse by magnetic resonance imaging and computed tomography. Physiol Genomics 2006; 24: 154–162.
Nieman BJ, Bock NA, Bishop J, Chen XJ, Sled JG, Rossant J et al. Magnetic resonance imaging for detection and analysis of mouse phenotypes. NMR Biomed 2005; 18: 447–468.
Collins DL, Neelin P, Peters TM, Evans AC . Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 1994; 18: 192–205.
Avants BB, Yushkevich P, Pluta J, Minkoff D, Korczykowski M, Detre J et al. The optimal template effect in hippocampus studies of diseased populations. Neuroimage 2010; 49: 2457–2466.
Dorr AE, Lerch JP, Spring S, Kabani N, Henkelman RM . High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6 J mice. Neuroimage 2008; 42: 60–69.
Suzuki R, Shimodaira H . Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 2006; 22: 1540–1542.
Courchesne E, Campbell K, Solso S . Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res 2011; 1380: 138–145.
Raznahan A, Wallace GL, Antezana L, Greenstein D, Lenroot R, Thurm A et al. Compared to what? Early brain overgrowth in autism and the perils of population norms. Biol Psychiatry 2013; 74: 563–575.
Gleave JA, Wong MD, Dazai J, Altaf M, Henkelman RM, Lerch JP et al. Neuroanatomical phenotyping of the mouse brain with three-dimensional autofluorescence imaging. Physiol Genomics 2012; 44: 778–785.
Toal F, Daly EM, Page L, Deeley Q, Hallahan B, Bloemen O et al. Clinical and anatomical heterogeneity in autistic spectrum disorder: a structural MRI study. Psychol Med 2010; 40: 1171–1181.
Amaral DG, Schumann CM, Nordahl CW . Neuroanatomy of autism. Trends Neurosci 2008; 31: 137–145.
Stanfield AC, McIntosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM . Towards a neuroanatomy of autism: A systematic review and meta-analysis of structural magnetic resonance imaging studies. Eur Psychiatry 2008; 23: 289–299.
Kurth F, Narr KL, Woods RP, O'Neill J, Alger JR, Caplan R et al. Diminished gray matter within the hypothalamus in autism disorder: a potential link to hormonal effects? Biol Psychiatry 2011; 70: 278–282.
Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ et al. Consensus paper: pathological role of the cerebellum in autism. Cerebellum 2012; 11: 777–807.
Kana RK, Libero LE, Moore MS . Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys Life Rev 2011; 8: 410–437.
Ey E, Leblond CS, Bourgeron T . Behavioral profiles of mouse models for autism spectrum disorders. Autism Res 2011; 4: 5–16.
Bailey KR, Rustay NR, Crawley JN . Behavioral phenotyping of transgenic and knockout mice: practical concerns and potential pitfalls. ILAR J 2006; 47: 124–131.
Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A et al. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav 2009; 8: 416–425.
Saywell V, Viola A, Confort-Gouny S, Le Fur Y, Villard L, Cozzone PJ . Brain magnetic resonance study of Mecp2 deletion effects on anatomy and metabolism. Biochem Biophys Res Commun 2006; 340: 776–783.
Ward BC, Agarwal S, Wang K, Berger-Sweeney J, Kolodny NH . Longitudinal brain MRI study in a mouse model of Rett Syndrome and the effects of choline. Neurobiol Dis 2008; 31: 110–119.
Dodero L, Damiano M, Galbusera A, Bifone A, Tsaftsaris SA, Scattoni ML et al. Neuroimaging evidence of major morpho-anatomical and functional abnormalities in the BTBR T+TF/J mouse model of autism. PLoS ONE 2013; 8: e76655.
Portmann T, Yang M, Mao R, Panagiotakos G, Ellegood J, Dolen G et al. Behavioral abnormalities and circuit defects in the basal ganglia of a mouse model of 16p11.2 deletion syndrome. Cell Rep 2014; 7: 1077–1092.
Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG et al. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron 2012; 74: 49–56.
Acknowledgements
This work was primarily funded by the Canadian Institute for Health Research (CIHR) and the Ontario Brain Institute (OBI). JE received salary support from the Ontario Mental Health Foundation (OHMF) and RMH holds a Canada Research Chair.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
EA has received consultation fees from Novartis and Seaside therapeutics, and has an unrestricted grant from Sanofi Canada. JV-VW receives research funding from Seaside Therapeutics, Novartis, Roche Pharmaceuticals, Forest, Sunovion and SynapDx and sits on the advisory board for Novartis and Roche Pharmaceuticals. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Ellegood, J., Anagnostou, E., Babineau, B. et al. Clustering autism: using neuroanatomical differences in 26 mouse models to gain insight into the heterogeneity. Mol Psychiatry 20, 118–125 (2015). https://doi.org/10.1038/mp.2014.98
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2014.98
This article is cited by
-
Peripheral oxytocin levels are linked to hypothalamic gray matter volume in autistic adults: a cross-sectional secondary data analysis
Scientific Reports (2024)
-
Dissecting 16p11.2 hemi-deletion to study sex-specific striatal phenotypes of neurodevelopmental disorders
Molecular Psychiatry (2024)
-
Mice with an autism-associated R451C mutation in neuroligin-3 show intact attention orienting but atypical responses to methylphenidate and atomoxetine in the mouse-Posner task
Psychopharmacology (2024)
-
Peripheral blood DNA methylation and neuroanatomical responses to HDACi treatment that rescues neurological deficits in a Kabuki syndrome mouse model
Clinical Epigenetics (2023)
-
Cntnap2-dependent molecular networks in autism spectrum disorder revealed through an integrative multi-omics analysis
Molecular Psychiatry (2023)