Abstract
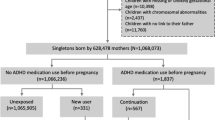
Previous studies suggested that risk for Autism Spectrum Disorder (ASD) may be increased in children exposed to antidepressants during the prenatal period. The disease specificity of this risk has not been addressed and the possibility of confounding has not been excluded. Children with ASD or attention-deficit hyperactivity disorder (ADHD) delivered in a large New England health-care system were identified from electronic health records (EHR), and each diagnostic group was matched 1:3 with children without ASD or ADHD. All children were linked with maternal health data using birth certificates and EHRs to determine prenatal medication exposures. Multiple logistic regression was used to examine association between prenatal antidepressant exposures and ASD or ADHD risk. A total of 1377 children diagnosed with ASD and 2243 with ADHD were matched with healthy controls. In models adjusted for sociodemographic features, antidepressant exposure prior to and during pregnancy was associated with ASD risk, but risk associated with exposure during pregnancy was no longer significant after controlling for maternal major depression (odds ratio (OR) 1.10 (0.70–1.70)). Conversely, antidepressant exposure during but not prior to pregnancy was associated with ADHD risk, even after adjustment for maternal depression (OR 1.81 (1.22–2.70)). These results suggest that the risk of autism observed with prenatal antidepressant exposure is likely confounded by severity of maternal illness, but further indicate that such exposure may still be associated with ADHD risk. This risk, modest in absolute terms, may still be a result of residual confounding and must be balanced against the substantial consequences of untreated maternal depression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Investigators AaDDMNSYP Prevalence of Autism Spectrum Disorders — Autism and Developmental Disabilities Monitoring Network. MMWR Surveill Summ 2012; 61: 1–19.
Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 1995; 25: 63–77.
Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 68: 1095–1102.
Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA . Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 2004; 306: 879–881.
Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V . Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011; 68: 1104–1112.
Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C . Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ 2013; 346: f2059.
Hviid A, Melbye M, Pasternak B . Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N Engl J Med 2013; 369: 2406–2415.
Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006; 295: 499–507.
Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C . Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry 2006; 63: 898–906.
Daniels JL, Forssen U, Hultman CM, Cnattingius S, Savitz DA, Feychting M et al. Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics 2008; 121: e1357–e1362.
Piven J, Palmer P . Psychiatric disorder and the broad autism phenotype: evidence from a family study of multiple-incidence autism families. Am J Psychiatry 1999; 156: 557–563.
Figueroa R . Use of antidepressants during pregnancy and risk of attention-deficit/hyperactivity disorder in the offspring. J Dev Behav Pediatr 2010; 31: 641–648.
Laugesen K, Olsen MS, Telen Andersen AB, Froslev T, Sorensen HT . In utero exposure to antidepressant drugs and risk of attention deficit hyperactivity disorder: a nationwide Danish cohort study. BMJ Open 2013; 3: e003507.
Murphy SN, Mendis M, Hackett K, Kuttan R, Pan W, Phillips LC et al. Architecture of the open-source clinical research chart from Informatics for Integrating Biology and the Bedside. AMIA Annu Symp Proc 2007; 548–552.
Murphy S, Churchill S, Bry L, Chueh H, Weiss S, Lazarus R et al. Instrumenting the health care enterprise for discovery research in the genomic era. Genome Res 2009; 19: 1675–1681.
Murphy SN, Weber G, Mendis M, Gainer V, Chueh HC, Churchill S et al. Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2). J Am Med Inform Assoc 17: 124–130.
Castro VM, Gallagher PJ, Clements CC, Murphy SN, Gainer VS, Fava M et al. Incident user cohort study of risk for gastrointestinal bleed and stroke in individuals with major depressive disorder treated with antidepressants. BMJ Open 2012; 2: e000544.
Owens MJ, Morgan WN, Plott SJ, Nemeroff CB . Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther 1997; 283: 1305–1322.
Schieve LA, Tian LH, Baio J, Rankin K, Rosenberg D, Wiggins L et al. Population attributable fractions for three perinatal risk factors for autism spectrum disorders, 2002 and 2008 autism and developmental disabilities monitoring network. Ann Epidemiol 2014; 24: 260–266.
Lash TL Fox MP Fink AK . A Guide to Implementing Quantitative Bias Analysis. In Gail M, Krickeberg K, Samet J, Tsiatis A, Wong W (eds). Applying Quantitative Bias Analysis to Epidemiologic Data, 1st edn, Springer: New York, NY, 2009. pp 215–220.
Acknowledgements
This work was supported through funding from the National Institute of Mental Health (5R01MH100286–02). Dr Perlis is supported by NIMH R01MH086026 and by the Stanley Center for Psychiatric Research. The i2b2 platform (PI: Kohane) is supported by award number 2U54LM008748 from the NIH/National Library of Medicine. We express our gratitude to the staff at the Massachusetts Registry of Vital Records and Statistics including Kevin Foster and Dean DiMartino.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Perlis has received consulting fees or served on scientific advisory boards for Genomind, Healthrageous, Pamlab, Perfect Health, Pfizer, Proteus Biomedical, Psybrain and RIDventures, and received patent fees/royalties from Concordant Rater Systems (now UBC/Medco).
Dr Smoller is a member of the Scientific Advisory Board of Psybrain, Inc.
Dr Fava reports the following: Research Support: Abbott Laboratories, Alkermes, Aspect Medical Systems, Astra-Zeneca, Bristol-Myers Squibb Company, Cephalon, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithkline, J & J Pharmaceuticals, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Novartis, Organon Inc., PamLab, LLC, Pfizer Inc, Pharmavite, Roche, Sanofi/Synthelabo, Solvay Pharmaceuticals, Inc., Wyeth-Ayerst Laboratories. Advisory/Consulting: Aspect Medical Systems, Astra-Zeneca, Bayer AG, Biovail Pharmaceuticals, Inc., BrainCells, Inc. Bristol-Myers Squibb Company, Cephalon, Compellis, Cypress Pharmaceuticals, Dov Pharmaceuticals, Eli Lilly & Company, EPIX Pharmaceuticals, Fabre-Kramer Pharmaceuticals, Inc., Forest Pharmaceuticals Inc., GlaxoSmithkline, Grunenthal GmBH, Janssen Pharmaceutica, Jazz Pharmaceuticals, J & J Pharmaceuticals, Knoll Pharmaceutical Company, Lundbeck, MedAvante, Inc., Neuronetics, Novartis, Nutrition 21, Organon Inc., PamLab, LLC, Pfizer Inc, PharmaStar, Pharmavite, Roche, Sanofi/Synthelabo, Sepracor, Solvay Pharmaceuticals, Inc., Somaxon, Somerset Pharmaceuticals, Wyeth-Ayerst Laboratories. Speaking: Astra-Zeneca, Boehringer-Ingelheim, Bristol-Myers Squibb Company, Cephalon, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithkline, Novartis, Organon Inc., Pfizer Inc, PharmaStar, Wyeth-Ayerst Laboratories. Equity Holdings: Compellis, MedAvante. Royalty/patent, other income: none.
The remaining authors declare no conflict of interests.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Clements, C., Castro, V., Blumenthal, S. et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 20, 727–734 (2015). https://doi.org/10.1038/mp.2014.90
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2014.90
This article is cited by
-
Should Antidepressants be Avoided in Pregnancy?
Drug Safety (2023)
-
Prenatal Antidepressant Exposures and Autism Spectrum Disorder or Traits: A Retrospective, Multi-Cohort Study
Research on Child and Adolescent Psychopathology (2023)
-
Prenatal risk factors and genetic causes of ADHD in children
World Journal of Pediatrics (2022)
-
A Systematic Review and Meta-analysis of Prenatal, Birth, and Postnatal Factors Associated with Attention-Deficit/Hyperactivity Disorder in Children
Prevention Science (2022)
-
Selective Serotonin Reuptake Inhibitors (SSRIs) in Pregnancy: An Updated Review on Risks to Mother, Fetus, and Child
Current Psychiatry Reports (2022)