Abstract
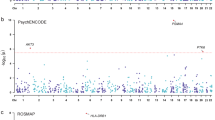
The N-methyl-d-aspartate receptor (NMDAR) coagonists glycine, d-serine and l-proline play crucial roles in NMDAR-dependent neurotransmission and are associated with a range of neuropsychiatric disorders. We conducted the first genome-wide association study of concentrations of these coagonists and their enantiomers in plasma and cerebrospinal fluid (CSF) of human subjects from the general population (N=414). Genetic variants at chromosome 22q11.2, located in and near PRODH (proline dehydrogenase), were associated with l-proline in plasma (β=0.29; P=6.38 × 10−10). The missense variant rs17279437 in the proline transporter SLC6A20 was associated with l-proline in CSF (β=0.28; P=9.68 × 10−9). Suggestive evidence of association was found for the d-serine plasma-CSF ratio at the d-amino-acid oxidase (DAO) gene (β=−0.28; P=9.08 × 10−8), whereas a variant in SRR (that encodes serine racemase and is associated with schizophrenia) constituted the most strongly associated locus for the l-serine to d-serine ratio in CSF. All these genes are highly expressed in rodent meninges and choroid plexus, anatomical regions relevant to CSF physiology. The enzymes and transporters they encode may be targeted to further construe the nature of NMDAR coagonist involvement in NMDAR gating. Furthermore, the highlighted genetic variants may be followed up in clinical populations, for example, schizophrenia and 22q11 deletion syndrome. Overall, this targeted metabolomics approach furthers the understanding of NMDAR coagonist concentration variability and sets the stage for non-targeted CSF metabolomics projects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Henneberger C, Papouin T, Oliet SH, Rusakov DA . Long-term potentiation depends on release of D-serine from astrocytes. Nature 2010; 463: 232–236.
Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M et al. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 2012; 150: 633–646.
Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT et al. Glycine binding primes NMDA receptor internalization. Nature 2003; 422: 302–307.
Sakata K, Fukushima T, Minje L, Ogurusu T, Taira H, Mishina M et al. Modulation by L- and D-isoforms of amino acids of the L-glutamate response of N-methyl-D-aspartate receptors. Biochemistry 1999; 38: 10099–10106.
McBain CJ, Kleckner NW, Wyrick S, Dingledine R . Structural requirements for activation of the glycine coagonist site of N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Mol Pharmacol 1989; 36: 556–565.
Martin D, Ault B, Nadler JV . NMDA receptor-mediated depolarizing action of proline on CA1 pyramidal cells. Eur J Pharmacol 1992; 219: 59–66.
Henzi V, Reichling DB, Helm SW, MacDermott AB . L-proline activates glutamate and glycine receptors in cultured rat dorsal horn neurons. Mol Pharmacol 1992; 41: 793–801.
Kleckner NW, Dingledine R . Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science 1988; 241: 835–837.
Clelland CL, Read LL, Baraldi AN, Bart CP, Pappas CA, Panek LJ et al. Evidence for association of hyperprolinemia with schizophrenia and a measure of clinical outcome. Schizophr Res 2011; 131: 139–145.
Jacquet H, Demily C, Houy E, Hecketsweiler B, Bou J, Raux G et al. Hyperprolinemia is a risk factor for schizoaffective disorder. Mol Psychiatry 2005; 10: 479–485.
Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N et al. Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry 2003; 60: 572–576.
Bendikov I, Nadri C, Amar S, Panizzutti R, De Miranda J, Wolosker H et al. A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophr Res 2007; 90: 41–51.
Frye MA, Tsai GE, Huggins T, Coyle JT, Post RM . Low cerebrospinal fluid glutamate and glycine in refractory affective disorder. Biol Psychiatry 2007; 61: 162–166.
Luykx JJ, Bakker SC, van Boxmeer L, Vinkers CH, Smeenk HE, Visser WF et al. D-amino acid aberrations in cerebrospinal fluid and plasma of smokers. Neuropsychopharmacology 2013; 38: 2019–2026.
Brouwer A, Luykx JJ, van Boxmeer L, Bakker SC, Kahn RS . NMDA-receptor coagonists in serum, plasma, and cerebrospinal fluid of schizophrenia patients: a meta-analysis of case-control studies. Neurosci Biobehav Rev 2013; 37: 1587–1596.
Goff DC, Tsai G, Levitt J, Amico E, Manoach D, Schoenfeld DA et al. A placebo-controlled trial of D-cycloserine added to conventional neuroleptics in patients with schizophrenia. Arch Gen psychiatry 1999; 56: 21–27.
Heresco-Levy U, Ermilov M, Lichtenberg P, Bar G, Javitt DC . High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia. Biol Psychiatry 2004; 55: 165–171.
Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Horowitz A, Kelly D . Double-blind, placebo-controlled, crossover trial of glycine adjuvant therapy for treatment-resistant schizophrenia. Br J Psychiatry 1996; 169: 610–617.
Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M . Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry 1999; 56: 29–36.
Tsai G, Yang P, Chung LC, Lange N, Coyle JT . D-serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry 1998; 44: 1081–1089.
Tsai GE, Yang P, Chang YC, Chong MY . D-alanine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry 2006; 59: 230–234.
Labrie V, Wong AH, Roder JC . Contributions of the D-serine pathway to schizophrenia. Neuropharmacology 2012; 62: 1484–1503.
Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry 2006; 63: 298–304.
Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P et al. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry 2008; 63: 544–549.
Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA et al. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry 2010; 67: 365–370.
Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry 2008; 165: 335–341.
Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry 2007; 62: 835–838.
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511: 421–427.
Kettunen J, Tukiainen T, Sarin AP, Ortega-Alonso A, Tikkanen E, Lyytikainen LP et al. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nature Genet 2012; 44: 269–276.
Suhre K, Wallaschofski H, Raffler J, Friedrich N, Haring R, Michael K et al. A genome-wide association study of metabolic traits in human urine. Nature Genet 2011; 43: 565–569.
Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 2011; 69: 893–905.
Fuchs SA, Berger R, de Koning TJ . D-serine: the right or wrong isoform? Brain Res 2011; 1401: 104–117.
Ma TM, Abazyan S, Abazyan B, Nomura J, Yang C, Seshadri S et al. Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion. Mol Psychiatry 2012; 18: 557–567.
Wolosker H, Blackshaw S, Snyder SH . Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci USA 1999; 96: 13409–13414.
Wolosker H, Sheth KN, Takahashi M, Mothet JP, Brady RO Jr, Ferris CD et al. Purification of serine racemase: biosynthesis of the neuromodulator D-serine. Proc Natl Acad Sci USA 1999; 96: 721–725.
Boks MP, Rietkerk T, van de Beek MH, Sommer IE, de Koning TJ, Kahn RS . Reviewing the role of the genes G72 and DAAO in glutamate neurotransmission in schizophrenia. Eur Neuropsychopharmacol 2007; 17: 567–572.
Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011; 17: 448–453.
Luykx JJ, Vinkers CH, Bakker SC, Visser WF, van Boxmeer L, Strengman E et al. A common variant in ERBB4 regulates GABA concentrations in human cerebrospinal fluid. Neuropsychopharmacology 2012; 37: 2088–2092.
Luykx JJ, Bakker SC, Lentjes E, Neeleman M, Strengman E, Mentink L et al. Genome-wide association study of monoamine metabolite levels in human cerebrospinal fluid. Mol Psychiatry 2014; 19: 228–234.
Visser WF, Verhoeven-Duif NM, Ophoff R, Bakker S, Klomp LW, Berger R et al. A sensitive and simple ultra-high-performance-liquid chromatography-tandem mass spectrometry based method for the quantification of d-amino acids in body fluids. J Chromatogr A 1218: 7130–7136.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
Browning SR, Browning BL . Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am J Hum Genet 2007; 81: 1084–1097.
Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR . Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nature Genet 2012; 44: 955–959.
Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR . MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol 2010; 34: 816–834.
Petersen AK, Krumsiek J, Wagele B, Theis FJ, Wichmann HE, Gieger C et al. On the hypothesis-free testing of metabolite ratios in genome-wide and metabolome-wide association studies. BMC Bioinformatics 2012; 13: 120.
Gieger C, Geistlinger L, Altmaier E, Hrabe de Angelis M, Kronenberg F, Meitinger T et al. Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLoS Genet 2008; 4: e1000282.
Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J et al. An atlas of genetic influences on human blood metabolites. Nature Genet 2014; 46: 543–550.
Nicholson G, Rantalainen M, Li JV, Maher AD, Malmodin D, Ahmadi KR et al. A genome-wide metabolic QTL analysis in Europeans implicates two loci shaped by recent positive selection. PLoS Genet 2011; 7: e1002270.
Suhre K, Shin SY, Petersen AK, Mohney RP, Meredith D, Wagele B et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011; 477: 54–60.
Guilmatre A, Legallic S, Steel G, Willis A, Di Rosa G, Goldenberg A et al. Type I hyperprolinemia: genotype/phenotype correlations. Hum Mutat 2010; 31: 961–965.
Bender HU, Almashanu S, Steel G, Hu CA, Lin WW, Willis A et al. Functional consequences of PRODH missense mutations. Am J Hum Genet 2005; 76: 409–420.
Diez-Roux G, Banfi S, Sultan M, Geffers L, Anand S, Rozado D et al. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol 2011; 9: e1000582.
Tanner JJ . Structural biology of proline catabolism. Amino Acids 2008; 35: 719–730.
Magdaleno S, Jensen P, Brumwell CL, Seal A, Lehman K, Asbury A et al. BGEM: an in situ hybridization database of gene expression in the embryonic and adult mouse nervous system. PLoS Biol 2006; 4: e86.
Visel A, Thaller C, Eichele G . GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res 2004; 32: D552–D556.
Broer S, Bailey CG, Kowalczuk S, Ng C, Vanslambrouck JM, Rodgers H et al. Iminoglycinuria and hyperglycinuria are discrete human phenotypes resulting from complex mutations in proline and glycine transporters. J Clin Invest 2008; 118: 3881–3892.
Dunlop DS, Neidle A, McHale D, Dunlop DM, Lajtha A . The presence of free D-aspartic acid in rodents and man. Biochem Biophys Res Commun 1986; 141: 27–32.
Burnet PW, Eastwood SL, Bristow GC, Godlewska BR, Sikka P, Walker M et al. D-amino acid oxidase activity and expression are increased in schizophrenia. Mol Psychiatry 2008; 13: 658–660.
De Miranda J, Santoro A, Engelender S, Wolosker H . Human serine racemase: moleular cloning, genomic organization and functional analysis. Gene 2000; 256: 183–188.
Raux G, Bumsel E, Hecketsweiler B, van Amelsvoort T, Zinkstok J, Manouvrier-Hanu S et al. Involvement of hyperprolinemia in cognitive and psychiatric features of the 22q11 deletion syndrome. Hum Mol Genet 2007; 16: 83–91.
Vorstman JA, Morcus ME, Duijff SN, Klaassen PW, Heineman-de Boer JA, Beemer FA et al. The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry 2006; 45: 1104–1113.
Sullivan PF, Daly MJ, O'Donovan M . Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet 2012; 13: 537–551.
Oresic M, Tang J, Seppanen-Laakso T, Mattila I, Saarni SE, Saarni SI et al. Metabolome in schizophrenia and other psychotic disorders: a general population-based study. Genome Med 2011; 3: 19.
Acknowledgements
We thank drs. JTA Knape, P Vaessen and P Keijzers for their valuable assistance in developing our CSF sampling protocol in the operating rooms and conducting the CSF samplings. We thank K van Eijk and J van Setten for their assistance in developing genetic quality control standards.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Rights and permissions
About this article
Cite this article
Luykx, J., Bakker, S., Visser, W. et al. Genome-wide association study of NMDA receptor coagonists in human cerebrospinal fluid and plasma. Mol Psychiatry 20, 1557–1564 (2015). https://doi.org/10.1038/mp.2014.190
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2014.190
This article is cited by
-
Novel Proline Transporter Inhibitor (LQFM215) Presents Antipsychotic Effect in Ketamine Model of Schizophrenia
Neurochemical Research (2024)
-
Genome-wide association analyses of symptom severity among clozapine-treated patients with schizophrenia spectrum disorders
Translational Psychiatry (2022)
-
Blood levels of D-amino acid oxidase vs. D-amino acids in reflecting cognitive aging
Scientific Reports (2017)