Abstract
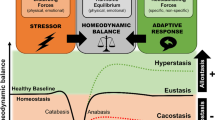
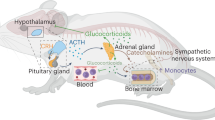
Stressors are imminent or perceived challenges to homeostasis. The stress response is an innate, stereotypic, adaptive response to stressors that has evolved in the service of restoring the nonstressed homeostatic set point. It is encoded in specific neuroanatomical sites that activate a specific repertoire of cognitive, behavioral and physiologic phenomena. Adaptive responses, though essential for survival, can become dysregulated and result in disease. A clear example is autoimmune disease. I postulate that depression, like autoimmunity, represents a dysregulated adaptive response: a stress response that has gone awry. The cardinal manifestation of the normal stress response is anxiety. Cognitive programs shift from complex associative operations to rapid retrieval of unconscious emotional memories acquired during prior threatening situations. These emerge automatically to promote survival. To prevent distraction during stressful situations, the capacity to seek and experience pleasure is reduced, food intake is diminished and sexual activity and sleep are held in abeyance. Monoamines, cytokines, glutamate, GABA and other central mediators have key roles in the normal stress response. Many central loci are involved. The subgenual prefrontal cortex restrains the amygdala, the corticotropin-releasing hormone/hypothalamic–pituitary–adrenal (CRH/HPA) axis and the sympathomedullary system. The function of the subgenual prefrontal cortex is moderately diminished during normal stress to disinhibit these loci. This disinhibition promotes anxiety and physiological hyperarousal, while diminishing appetite and sleep. The dorsolateral prefrontal cortex is downregulated, diminishing cognitive regulation of anxiety. The nucleus accumbens is also downregulated, to reduce the propensity for distraction by pleasurable stimuli or the capacity to experience pleasure. Insulin resistance, inflammation and a prothrombotic state acutely emerge. These provide increased glucose for the brain and establish premonitory, proinflammatory and prothrombotic states in anticipation of either injury or hemorrhage during a threatening situation. Essential adaptive intracellular changes include increased neurogenesis, enhancement of neuroplasticity and deployment of a successful endoplasmic reticulum stress response. In melancholic depression, the activities of the central glutamate, norepinephrine and central cytokine systems are significantly and persistently increased. The subgenual prefrontal cortex is functionally impaired, and its size is reduced by as much as 40%. This leads to sustained anxiety and activations of the amygdala, CRH/HPA axis, the sympathomedullary system and their sequella, including early morning awakening and loss of appetite. The sustained activation of the amygdala, in turn, further activates stress system neuroendocrine and autonomic functions. The activity of the nucleus accumbens is further decreased and anhedonia emerges. Concomitantly, neurogenesis and neuroplasticity fall significantly. Antidepressants ameliorate many of these processes. The processes that lead to the behavioral and physiological manifestations of depressive illness produce a significant decrease in lifespan, and a doubling of the incidence of premature coronary artery disease. The incidences of premature diabetes and osteoporosis are also substantially increased. Six physiological processes that occur during stress and that are markedly increased in melancholia set into motion six different mechanisms to produce inflammation, as well as sustained insulin resistance and a prothrombotic state. Clinically, melancholic and atypical depression seem to be antithesis of one another. In melancholia, depressive systems are at their worst in the morning when arousal systems, such as the CRH/HPA axis and the noradrenergic systems, are at their maxima. In atypical depression, depressive symptoms are at their worst in the evening, when these arousal systems are at their minima. Melancholic patients experience anorexia and insomnia, whereas atypical patients experience hyperphagia and hypersomnia. Melancholia seems like an activation and persistence of the normal stress response, whereas atypical depression resembles a stress response that has been excessively inhibited. It is important that we stratify clinical studies of depressed patients to compare melancholic and atypical subtypes and establish their differential pathophysiology. Overall, it is important to note that many of the major mediators of the stress response and melancholic depression, such as the subgenual prefrontal cortex, the amygdala, the noradrenergic system and the CRH/HPA axis participate in multiple reinforcing positive feedback loops. This organization permits the establishment of the markedly exaggerated, persistent elevation of the stress response seen in melancholia. Given their pronounced interrelatedness, it may not matter where in this cascade the first abnormality arises. It will spread to the other loci and initiate each of their activations in a pernicious vicious cycle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gold PW, Goodwin FK, Chrousos GP . Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress (1). N Engl J Med 1988; 31: 348–353.
Gold PW, Goodwin FK, Chrousos GP . Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress (2). N Engl J Med 1988; 319: 413–420.
Chrousos GP, Gold PW . The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 1992; 267: 1244–1252.
Arnsten AF . Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 2009; 10: 410–422.
McGaugh JL . The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 2004; 27: 1–28.
Uchida Y, Takeshita K, Yamamoto K, Kikuchi R, Nakayama T, Nomura M et al. Stress augments insulin resistance and prothrombotic state: role of visceral adipose-derived monocyte chemoattractant protein-1. Diabetes 2012; 61: 1552–1561.
Steptoe A, Hamer M, Chida Y . The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun 2007; 21: 901–912.
Duman RS, Li N . A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci 2012; 367: 2475–2484.
Pittenger C, Duman RS . Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 2008; 33: 88–109.
Hotamisligil GS . Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010; 140: 900–917.
Gold PW, Licinio J, Pavlatou MG . Pathological parainflammation and endoplasmic reticulum stress in depression: potential translational targets through the CNS insulin, klotho and PPAR-gamma systems. Mol Psychiatry 2013; 18: 154–165.
Simpson JR Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME . Emotion-induced changes in humanmedial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci USA 2001; 98: 688–693.
Drevets WC, Savitz J, Trimble M . The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 2008; 13: 663–681.
Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA . A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 2011; 12: 585–601.
Price JL, Drevets WC . Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci 2012; 16: 61–71.
Berridge KC, Kringelbach ML . Neuroscience of affect: brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol 2013; 23: 294–303.
Diorio D, Viau V, Meaney MJ . The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci 1993; 13: 3839–3847.
Phelps EA, LeDoux JE . Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 2005; 48: 175–187.
Vale W, Spiess J, Rivier C, Rivier J . Characterization of a 41-residue hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981; 213: 1394–1397.
Ashton-Jomes G . An integrative theory of locus ceruleus-norepinphrine function: adaptive gain and optimal performance. Annu Rev Neurosci 2005; 28: 403–450.
Brown MR, Fisher LA, Spiess J, Rivier C, Rivier J, Vale WW . Corticotropin releasing factor: actions on the sympathetic nervous system and metabolism. Endocrinology 1982; 111: 928–931.
Karalis K, Sano H, Redwine J, Listwak S, Wilder RL, Chrousos GP . Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vitro. Science 1991; 254: 421–423.
Makino S, Gold PW, Schulkin J . Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Res 1994; 42: 25–28.
Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P et al. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci USA 2000; 97: 325–330.
Puig MV, Gulledge AT . Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol 2011; 44: 449–464.
Popoli M, Yan Z, McEwen BS, Sanacora G . The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 2012; 13: 22–37.
Spinedi E, Hadid R, Daneva T, Gaillard RC . Cytokines stimulate the CRH but not the vasopressin neuronal system: evidence for a median eminence site of interleukin-6 action. Neuroendocrinology 1992; 56: 46–53.
Papanicolaou DA, Petrides JS, Tsigos C, Bina S, Kalogeras KT, Wilder R et al. Exercise stimulates interleukin-6 secretion: inhibition by glucocorticoids and correlation with catecholamines. Am J Physiol 1996; 271: E601–E605.
Hotamisligil GS, Shargill NS, Spiegelman BM . Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259: 87–91.
Joseph L, Fink LM, Hauer-Jensen M . Cytokines in coagulationand thrombosis: a preclinical and clinical review. Blood Coagul Fibrinolysis 2002; 13: 105–116.
Weissman C . The metabolic response to stress: an overview and update. Anesthesiology 1990; 73: 308–327.
Hotamisligil GS . Inflammation and metabolic disorders. Nature 2006; 444: 860–867.
Miller AH, Maletic V, Raison CL . Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65: 732–741.
Autry AE, Monteggia LM . Brain-derived neurotrophic factor and neuropsychiatric disoders. Pharmacol Rev 2012; 64: 238–258.
Park H, Poo MM . Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 2013; 14: 7–23.
Greenberg ME, Xu B, Lu B, Hempstead BL . New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci 2009; 29: 12764–12767.
Duman RS . Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: stress and depression. Dialogues Clin Neurosci 2009; 11: 239–255.
Duman RS, Aghajanian GK . Synaptic dysfunction in depression: potential therapeutic targets. Science 2012; 338: 68–72.
Ming G-L, Song H . Adult neurogenesis in the mammalian brain. Neuron 2011; 70: 687–702.
Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 2011; 472: 466–470.
Snyder JS, Soumier A, Brewer M, Pickel j, Cameron HA . Hippocampal neurogenesis buffers stress responses and depressive behavior. Nature 2011; 476: 458–461.
Simmons D . Epigenetic influences and disease. Nat Educ 2008; 1: 6–8.
Nestler EJ . Stress makes its molecular mark. Nature 2012; 490: 171–172.
Gold PW, Wong ML, Goldstein DS, Gold HK, Ronsaville DS, Esler M et al. Cardiac implications of increased arterial entry and reversible 24-h central and peripheral norepinephrine levels in melancholia. Proc Natl Acad Sci USA 2005; 102: 8303–8308.
Gold PW, Loriaux DL, Roy A, Kling MA, Calabrese JR, Kellner CH et al. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing's disease. Pathophysiologic and diagnostic implications. N Engl J Med 1986; 314: 1329–1335.
Joseph-Vanderpool JR, Rosenthal NE, Chrousos GP, Wehr TA, Skwerer R, Kasper S et al. Abnormal pituitary-adrenal responses to corticotropin-releasing hormone in patients with seasonal affective disorder: clinical and pathophysiological implications. J Clin Endocrinol Metab 1991; 72: 1382–1387.
Gold PW, Chrousos GP . Melancholic and atypical subtypes of depression represent distinct pathophysiological entities: CRH, neural circuits, and the diathesis for anxiety and depression. Mol Psychiatry 2013; 18: 632–634.
Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW . Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry 2012; 18: 692–699.
Sternberg EM, Chrousos GP, Wilder RL, Gold PW . The stress response and the regulation of inflammatory disease. Ann Intern Med. 1992; 117: 854–866.
Withers AC, Tarasoff JM, Stewart JW . Is depression with atypical features associated with trauma history? J Clin Psychiatry 2013; 74: 500–506.
Spitz RA . Hospitalism; an inquiry into the genesis of psychiatric conditions in early childhood. Psychoanal Study Child 1945; 1: 53–74.
Zioli F . Maternal separation produces long-lasting changes in cortisol and behavior in rhesus monkeys. Proc Natl Acad Sci USA 2010; 108: 14312–14317.
Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997; 386: 824–827.
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C et al. Deep brain stimulation for treatment-resistant depression. Neuron 2005; 45: 651–660.
Disner SG, Beevers CG, Haigh EA, Beck AT . Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci 2011; 1: 467–477.
Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 2009; 166: 702–10.47.
Nauczyciel C, Robic S, Dondaine T, Verin M, Robert G, Drapier D et al. The nucleus accumbens: a target for deep brain stimulation in resistant major depressive disorder. Mol Psychiatry 2013; 17: 1–12.
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA et al. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63: 856–864.
Duman RS, Li N, Liu RJ, Duric V, Aghajanian G . Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 2012; 62: 35–41.
Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 2013; 38: 729–742.
Huang CC, Wei IH, Huang CL, Chen KT, Tsai MH, Tsai P et al. Inhibition of glycine transporter-I as a novel mechanism for the treatment of depression. Biol Psychiatry 2013; 74: 734–741.
Chen CH, Lee CS, Lee MM, Ouyang WC, Chen CC, Chong MY et al. Variant GADL1 and response to lithium therapy in bipolar I disorder. N Eng J Med 2014; 370: 119–128.
Nicholson A, Kuper H, Hemingway H . Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J 2006; 27: 2763–2774.
Jones BS, Mussolino ME . Symptoms of depression as a prospective factor for stroke. Psychosom Med 2000; 62: 463–471.
Knol MJ, Twisk JWR, Beekman ATF, Heine RJ, Snoek FJ, Pouwer F et al. Depression as a risk factor for the onset of type II diabetes. Diabetologia 2005; 49: 837–845.
de Jonge P, Alonso J, Stein DJ, Kiejna A, Aguilar-Gaxiola S, Viana MC et al. Associations between DSM-IV mental disordersand diabetes mellitus: a role for impulse control disorders and depression. Diabetologia 2014; 57: 699–707.
Michelson D, Stratakis C, Hill L, Reynolds J, Galliven E, Chrousos G et al. Bone mineral density in women with depression. N Engl J Med 1996; 335: 1176–1181.
Verhoeven JE, Revesz D, Epel ES, Lin J, Wolkowitz OM, Penninx BW . Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry 2013; 19: 895–901.
Heinrich PC, Castell JV, Andus T . Interleukin-6 and the acute phase response. Biochem J 1990; 265: 621–636.
Eskandari F, Mistry S, Martinez PE, Torvik S, Kotila C, Sebring N et al. Younger, premenopausal women with major depressive disorder have more abdominal fat and increased serum levels of prothrombotic factors: implications for greater cardiovascular risk. Metabolism 2005; 54: 918–924.
Okamura F, Tashiro A, Utumi A, Imai T, Suchi T, Tamura D et al. Insulin resistance in patients with depression and its changes during the clinical course of depression: minimal model analysis. Metabolism 2000; 4: 1255–1260.
Kling MA, Alesci S, Csako G, Costello R, Luckenbaugh DA, Bonne O et al. Sustained low-grade pro-inflammatory state in unmedicated, remitted women with major depressive disorder as evidenced by elevated serum levels of the acute phase proteins C-reactive protein and serum amyloid A. Biol Psychiatry 2006; 62: 309–313.
Bjorntorp P . Metabolic implications of body fat distribution. Diabetes Care 1991; 14: 1132–1143.
Fernandez-Real JM, Vayreda M, Richart C . Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab 2001; 86: 1154–1159.
Kapiotis S, Holzer G, Schaller G, Haumer M, Widhalm H, Weghuber D et al. A proinflammatory state is detectable in obese children and is accompanied by functional and morphological vascular changes. Arterioscler Thromb Vasc Biol 2006; 26: 2541–2546.
Hausberg M, Mark AL, Hoffman RP, Sinkey CA, Anderson EA . Dissociation of sympathoexcitatory and vasodilator actions of modestly elevated plasma insulin levels. J Hypertens 1995; 13: 1015–1021.
Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ . Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol 2010; 588: 3593–3603.
Medzhitov R . Origin and physiological roles of inflammation. Nature 2008; 454: 428–435.
Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry 2009; 65: 296–303.
Miller AH, Haroon E, Raison CL, Felger JC . Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 2013; 30: 297–306.
Raison CL, Capuron L, Miller AH . Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 2006; 27: 24–31.
Ida T, Hara M, Nakamura Y, Kozaki S, Tsunoda S, Ihara H . Cytokine-induced enhancement of calcium-dependent glutamate release from astrocytes mediated by nitric oxide. Neurosci Lett 2008; 432: 232–236.
Koo JW, Duman RS . Evidence for IL-1 receptor blockade as a therapeutic strategy for the treatment of depression. Curr Opin Investig Drugs 2009; 10: 664–671.
Ben Menachem-Zidon O, Goshen I, Kreisel T, Ben Menahem Y, Reinhartz E, Ben Hur T et al. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology 2008; 33: 2251–2262.
Pariante CM, Miller AH . Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry 2001; 49: 39187.
Duman RS, Heninger GR, Nestler EJ . A molecular and cellular theory of depression. Arch Gen Psychiatry 1997; 54: 597–606.
Karege F, Perret G, Schwald M, Bertschy G, Aubry JM . Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res 2002; 109: 143–148.
David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I et al. Neurogenesis-dependent and independent effects of fluoxetine in an animal models of anxiety/depression. Neuron 2009; 62: 479–493.
Santarelli L, Saxe M, Gross C . Requirment of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003; 301: 805–808.
Nestler EJ . Epigenetic mechanisms of depression. JAMA Psychiatry 2014; 71: 454–456.
Vialou V, Feng J, Robison AJ, Nestler EJ . Epigenetic mechanisms of depression and antidepressant action. Annu Rev Pharmacol Toxicol 2013; 53: 59–87.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Gold, P. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry 20, 32–47 (2015). https://doi.org/10.1038/mp.2014.163
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2014.163
This article is cited by
-
Kompetenzen in Psychotherapeutischer Medizin und Psychotherapie erwerben
psychopraxis. neuropraxis (2024)
-
Histone 3 Trimethylation Patterns are Associated with Resilience or Stress Susceptibility in a Rat Model of Major Depression Disorder
Molecular Neurobiology (2024)
-
The relationship between depression and benign prostatic hyperplasia in middle-aged and elderly men in India: a large-scale population study
BMC Public Health (2023)
-
PET imaging of animal models with depressive-like phenotypes
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Safety and efficacy of Ninjin’yoeito along with iron supplementation therapy for preoperative anemia, fatigue, and anxiety in patients with gynecological disease: an open-label, single-center, randomized phase-II trial
BMC Women's Health (2022)