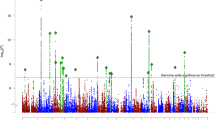
Abstract
After two decades of frustration, genetic studies of schizophrenia have entered an era of spectacular success. Advances in genotyping technologies and high throughput sequencing, increasing analytic rigour and collaborative efforts on a global scale have generated a profusion of new findings. The broad conclusions from these studies are threefold: (1) schizophrenia is a highly polygenic disorder with a complex array of contributing risk loci across the allelic frequency spectrum; (2) many psychiatric illnesses share risk genes and alleles, specifically, schizophrenia has substantial overlaps with bipolar disorder, intellectual disability, major depressive disorder and autism spectrum disorders; and (3) some convergent biological themes are emerging from studies of schizophrenia and related disorders. In this commentary, we focus on the very recent findings that have emerged in the past 12 months, and in particular, the areas of convergence that are beginning to emerge from multiple study designs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Owen MJ, O’Donovan MC, Gottesman II . Schizophrenia In Psychiatric Genetics and Genomics. OUP Oxford New Ed edition 2002 pp 247–266.
O’Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet 2008; 40: 1053–1055.
Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D et al. Common variants conferring risk of schizophrenia. Nature 2009; 460: 744–747.
Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 2009; 460: 753–757.
International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009; 460: 748–752.
Ikeda M, Aleksic B, Kinoshita Y, Okochi T, Kawashima K, Kushima I et al. Genome-wide association study of schizophrenia in a Japanese population. Biol Psychiatry 2011; 69: 472–478.
Hamshere M L, Walters JT, Smith R, Richards AL, Green E, Grozeva D et al. Genome-wide significant associations in schizophrenia to ITIH3/4, CACNA1C and SDCCAG8, and extensive replication of associations reported by the Schizophrenia PGC. Mol Psychiatry 2012; 18: 708–712 10.1038/mp.2012.67.
Genome-wide association study implicates HLA-C*01. 02 as a risk factor at the major histocompatibility complex locus in schizophrenia. Biol Psychiatry 2012; 72: 620–628.
Lencz T, Guha S, Liu C, Rosenfeld J, Mukherjee S, DeRosse P et al. Genome-wide association study implicates NDST3 in schizophrenia and bipolar disorder. Nat Commun 2013; 4: 2739.
Sullivan PF, Daly MJ, O’Donovan M . Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet 2012; 13: 537–551.
Ripke S, O'Dushlaine C, Chambert K, Moran JL, Kähler AK, Akterin S et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet 2013; 45: 1150–1159.
Green E K, Grozeva D, Jones I, Jones L, Kirov G, Caesar S et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry 2010; 15: 1016–1022.
Smoller JW et al. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 2013; 381: 1371–1379.
Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, Mowry BJ et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet 2013; 45: 984–994.
Yang J, Lee SH, Goddard ME, Visscher PM . GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet 2011; 88: 76–82.
Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci 1995; 92: 7612–7616.
Malhotra D, Sebat J . CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell 2012; 148: 1223–1241.
Xu B, Roos JL, Levy S, van Rensburg EJ, Gogos JA, Karayiorgou M et al. Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet 2008; 40: 880–885.
Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry 2012; 17: 142–153.
Rees E, Walters JT, Georgieva L, Isles AR, Chambert KD, Richards AL et al. Analysis of copy number variations at 15 schizophrenia-associated loci in a large, independent cohort. Br J Psychiatry 2013; 204: 108–114.
Szatkiewicz JP, O'Dushlaine C, Chen G, Chambert K, Moran JL, Neale BM et al. Copy number variation in schizophrenia in Sweden. Mol Psychiatry 2014; 19: 762–773.
Elia J, Glessner JT, Wang K, Takahashi N, Shtir CJ, Hadley D et al. Genome-wide copy number variation study associates metabotropic glutamate receptor gene networks with attention deficit hyperactivity disorder. Nat Genet 2012; 44: 78–84.
Cooper GM, Coe BP, Girirajan S, Rosenfeld JA, Vu TH, Baker C et al. A copy number variation morbidity map of developmental delay. Nat Genet 2011; 43: 838–846.
Williams NM, Franke B, Mick E, Anney RJ, Freitag CM, Gill M et al. Genome-wide analysis of copy number variants in attention deficit hyperactivity disorder: the role of rare variants and duplications at 15q13.3. Am J Psychiatry 2012; 169: 195–204.
Girirajan S, Eichler EE . Phenotypic variability and genetic susceptibility to genomic disorders. Hum Mol Genet 2010; 19: R176–R187.
Sahoo T, Theisen A, Rosenfeld JA, Lamb AN, Ravnan JB, Schultz RA et al. Copy number variants of schizophrenia susceptibility loci are associated with a spectrum of speech and developmental delays and behavior problems. Genet Med 2011; 13: 868–880.
Kirov G, Rees E, Walters JT, Escott-Price V, Georgieva L, Richards AL et al. The Penetrance of Copy Number Variations for Schizophrenia and Developmental Delay. Biol Psychiatry 2013; 75: 378–385.
Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science 2008; 320: 539–543.
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511: 421–427.
Hamshere ML, Walters JT, Smith R, Richards AL, Green E, Grozeva D et al. Genome-wide significant associations in schizophrenia to ITIH3/4, CACNA1C and SDCCAG8, and extensive replication of associations reported by the Schizophrenia PGC. Mol Psychiatry 2013; 18: 708–712.
Plenge RM, Scolnick EM, Altshuler D . Validating therapeutic targets through human genetics. Nat Rev Drug Discov 2013; 12: 581–594.
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 2012; 489: 57–74.
Carter CS, Bullmore ET, Harrison P . Is there a flame in the brain in psychosis? Biol Psychiatry 2014; 75: 258–259.
Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P et al. De novo mutations in schizophrenia implicate synaptic networks. Nature 2014; 506: 179–184.
Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature 2014; 506: 185–190.
Xu B, Ionita-Laza I, Roos JL, Boone B, Woodrick S, Sun Y et al. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat Genet 2012; 44: 1365–1369.
Girard SL, Gauthier J, Noreau A, Xiong L, Zhou S, Jouan L et al. Increased exonic de novo mutation rate in individuals with schizophrenia. Nat Genet 2011; 43: 860–863.
Gulsuner S, Walsh T, Watts AC, Lee MK, Thornton AM, Casadei S et al. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell 2013; 154: 518–529.
Neale BM, Kou Y, Liu L, Ma'ayan A, Samocha KE, Sabo A et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 2012; 485: 242–245.
O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 2012; 485: 246–250.
Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 2012; 485: 237–241.
Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J et al. De Novo Gene Disruptions in Children on the Autistic Spectrum. Neuron 2012; 74: 285–299.
Rauch A, Wieczorek D, Graf E, Wieland T, Endele S, Schwarzmayr T et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet 2012; 380: 1674–1682.
De Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T et al. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med 2012; 367: 1921–1929.
Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 2012; 488: 471–475.
McGrath JJ, Petersen L, Agerbo E, Mors O, Mortensen PB, Pedersen CB et al. A comprehensive assessment of parental age and psychiatric disorders. JAMA Psychiatry 2014; 71: 301–309.
McCarthy SE, Gillis J, Kramer M, Lihm J, Yoon S, Berstein Y et al. De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Mol Psychiatry 2014; 19: 652–658.
Craddock N, Owen MJ . The Kraepelinian dichotomy – going, going... but still not gone. Br J Psychiatry 2010; 196: 92–95.
Doherty JL, Owen MJ . The Research Domain Criteria: moving the goalposts to change the game. Br J Psychiatry 2014; 204: 171–173.
Doherty JL, Owen MJ . Genomic insights into the overlap between psychiatric disorders: implications for research and clinical practice. Genome Med 2014; 6: 29.
McCarroll SA, Hyman SE . Progress in the genetics of polygenic brain disorders: significant new challenges for neurobiology. Neuron 2013; 80: 578–587.
Gaj T, Gersbach CA, Barbas CF . ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 2013; 31: 397–405.
Zhang F, Wen Y, Guo X . CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet 2014; 23: R40–R46.
Linden DEJ . The challenges and promise of neuroimaging in psychiatry. Neuron 2012; 73: 8–22.
Acknowledgements
This work on schizophrenia is supported by MRC Centre (G0800509) and MRC Programme (G0801418) Grants, the European Community's Seventh Framework Programme (HEALTH-F2-2010-241909 (Project EU-GEI)) and the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no 279227.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declare no conflicts of intrest.
Rights and permissions
About this article
Cite this article
Kavanagh, D., Tansey, K., O'Donovan, M. et al. Schizophrenia genetics: emerging themes for a complex disorder. Mol Psychiatry 20, 72–76 (2015). https://doi.org/10.1038/mp.2014.148
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2014.148
This article is cited by
-
Common and rare variant associations with latent traits underlying depression, bipolar disorder, and schizophrenia
Translational Psychiatry (2023)
-
MAP2 is differentially phosphorylated in schizophrenia, altering its function
Molecular Psychiatry (2021)
-
Glutamate and microglia activation as a driver of dendritic apoptosis: a core pathophysiological mechanism to understand schizophrenia
Translational Psychiatry (2021)
-
Epigenetic mechanisms in schizophrenia and other psychotic disorders: a systematic review of empirical human findings
Molecular Psychiatry (2020)
-
GABAergic Abnormalities Associated with Sensorimotor Cortico-striatal Community Structural Deficits in ErbB4 Knockout Mice and First-Episode Treatment-Naïve Patients with Schizophrenia
Neuroscience Bulletin (2020)