Abstract
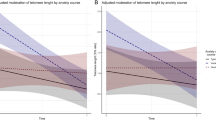
There is evidence that persistent psychiatric disorders lead to age-related disease and premature mortality. Telomere length has emerged as a promising biomarker in studies that test the hypothesis that internalizing psychiatric disorders are associated with accumulating cellular damage. We tested the association between the persistence of internalizing disorders (depression, generalized anxiety disorder and post-traumatic stress disorder) and leukocyte telomere length (LTL) in the prospective longitudinal Dunedin Study (n=1037). Analyses showed that the persistence of internalizing disorders across repeated assessments from ages 11 to 38 years predicted shorter LTL at age 38 years in a dose–response manner, specifically in men (β=−0.137, 95% confidence interval (CI): −0.232, −0.042, P=0.005). This association was not accounted for by alternative explanatory factors, including childhood maltreatment, tobacco smoking, substance dependence, psychiatric medication use, poor physical health or low socioeconomic status. Additional analyses using DNA from blood collected at two time points (ages 26 and 38 years) showed that LTL erosion was accelerated among men who were diagnosed with internalizing disorder in the interim (β=−0.111, 95% CI: −0.184, −0.037, P=0.003). No significant associations were found among women in any analysis, highlighting potential sex differences in internalizing-related telomere biology. These findings point to a potential mechanism linking internalizing disorders to accelerated biological aging in the first half of the life course, particularly in men. Because internalizing disorders are treatable, the findings suggest the hypothesis that treating psychiatric disorders in the first half of the life course may reduce the population burden of age-related disease and extend health expectancy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Vaupel JW. Biodemography of human ageing. Nature 2010; 464: 536–542.
Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science 2004; 305: 1736–1739.
Kirkwood TBL, Austad SN. Why do we age? Nature 2000; 408: 233–238.
Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav 2012; 106: 29–39.
Wellcome Trust. Ageing: can we stop the clock? Wellcome Trust, 2006.
Brayne C. The elephant in the room—healthy brains in later life, epidemiology and public health. Nat Rev Neurosci 2007; 8: 233–239.
Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis 2006; 3: A42.
Druss BG, Zhao L, Von Esenwein S, Morrato EH, Marcus SC. Understanding excess mortality in persons with mental illness 17-year follow up of a nationally representative US Survey. Med Care 2011; 49: 599–604.
Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Disord 2002; 72: 227–236.
Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol 2011; 108: 29–33.
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 593–602.
Epel ES Telomeres in a life-span perspective: a new ‘psychobiomarker’? Curr Dir Psychol Sci 2009; 18: 6–10.
Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011; 470: 359–365.
Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet 2012; 13: 693–704.
Cohen S, Janicki-Deverts D, Turner RB, Casselbrant ML, Li-Korotky HS, Epel ES et al. Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. JAMA 2013; 309: 699–705.
Kiecolt-Glaser JK, Jaremka LM, Derry HM, Glaser R. Telomere length: a marker of disease susceptibility? Brain Behav Immun 2013; 34: 29–30.
Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry 2006; 60: 432–435.
Hartmann N, Boehner M, Groenen F, Kalb R Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depress Anxiety 2010; 27: 1111–1116.
Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su YL et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress - preliminary findings. PLoS ONE 2011; 6: e17837.
O'Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry 2011; 70: 465–471.
Ladwig K-H, Brockhaus AC, Baumert J, Lukaschek K, Emeny RT, Kruse J et al. Posttraumatic stress disorder and not depression is associated with shorter leukocyte telomere length: findings from 3000 participants in the population-based KORA F4 Study. PLoS ONE 2013; 8: e64762.
Okereke OI, Prescott J, Wong JYY, Han JL, Rexrode KM, De Vivo I. High phobic anxiety is related to lower leukocyte telomere length in women. PLoS ONE 2012; 7: e40516.
Phillips AC, Robertson T, Carroll D, Der G, Shiels PG, McGlynn L et al. Do symptoms of depression predict telomere length? evidence from the West of Scotland Twenty-07 Study. Psychosom Med 2013; 75: 288–296.
Hoen PW, de Jonge P, Na BY, Farzaneh-Far R, Epel E, Lin J et al. Depression and leukocyte telomere length in patients with coronary heart disease: data from the Heart and Soul Study. Psychosom Med 2011; 73: 541–547.
Brady KT, Killeen TK, Brewerton T, Lucerini S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry 2000; 61 ((Suppl 7)): 22–32.
Sartor CE, Grant JD, Lynskey MT, McCutcheon VV, Waldron M, Statham DJ et al. Common heritable contributions to low-risk trauma, high-risk trauma, posttraumatic stress disorder, and major depression. Arch Gen Psychiatry 2012; 69: 293–299.
Eaton NR, Keyes KM, Krueger RF, Balsis S, Skodol AE, Markon KE et al. An invariant dimensional liability model of gender differences in mental disorder prevalence: evidence from a national sample. J Abnorm Psychol 2012; 121: 282–288.
Olff M, Langeland W, Draijer N, Gersons BP. Gender differences in posttraumatic stress disorder. Psychol Bull 2007; 133: 183–204.
Barrett EL, Richardson DS. Sex differences in telomeres and lifespan. Aging Cell 2011; 10: 913–921.
Chang CK, Hayes RD, Broadbent M, Fernandes AC, Lee W, Hotopf M et al. All-cause mortality among people with serious mental illness (SMI), substance use disorders, and depressive disorders in southeast London: a cohort study. BMC Psychiatry 2010; 10; doi:10.1186/1471-244X-10-77.
Caspi A, Moffitt TE, Thornton A, Freedman D, Amell JW, Harrington H et al. The life history calendar: a research and clinical assessment method for collecting retrospective event-history data. Int J Method Psych 1996; 6: 101–114.
Costello A, Edelbrock C, Kalas R, Kessler M, Klaric S. NIMH Diagnostic Interview for Children: Child Version. National Institute of Mental Health: Rockville, MD, USA, 1982.
Robins L, Cottler L, Bucholz K, Compton W. Diagnostic Interview Schedule for DSM-IV (DIS-IV) 1995.
Moffitt TE, Caspi A, Taylor A, Kokaua J, Milne BJ, Polanczyk G et al. How common are common mental disorders? evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychol Med 2010; 40: 899–909.
Bowtell DD. Rapid isolation of eukaryotic DNA. Anal Biochem 1987; 162: 463–465.
Jeanpierre M. A rapid method for the purification of DNA from blood. Nucleic Acids Res 1987; 15: 9611.
Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002; 30: e47.
Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry 2013; 18: 576–581.
Selvin S. Statistical Analysis of Epidemiologic Data. Oxford University Press: New York, NY, USA, 2004.
Steenstrup T, Hjelmborg JV, Kark JD, Christensen K, Aviv A. The telomere lengthening conundrum—artifact or biology? Nucleic Acids Res 2013; 41: e131.
Verhulst S, Aviv A, Benetos A, Berenson GS, Kark JD. Do leukocyte telomere length dynamics depend on baseline telomere length? an analysis that corrects for 'regression to the mean'. Eur J Epidemiol 2013; 28: 859–866.
Epel E. How ‘reversible’ is telomeric aging? Cancer Prev Res 2012; 5: 1163–1168.
Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol 2005; 69: 113–132.
Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71: 171–186.
Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M et al. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol 2002; 22: 438–442.
von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci 2002; 27: 339–344.
O'Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K et al. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS ONE 2011; 6: e19687.
Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun 2008; 22: 600–605.
Vina J, Sastre J, Pallardo' F, Borras C. Mitochondrial theory of aging: importance to explain why females live longer than males. Antioxid Redox Sign 2003; 5: 549–556.
Borras C, Sastre J, Garcia-Sala D, Lloret A, Pallardo FV, Vina J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radical Bio Med 2003; 34: 546–552.
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013; 153: 1194–1217.
Misiti S, Nanni S, Fontemaggi G, Cong YS, Wen JP, Hirte HW et al. Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells. Mol Cell Biol 2000; 20: 3764–3771.
Bayne S, Jones MEE, Ll H, Liu JP. Potential roles for estrogen regulation of telomerase activity in aging. Ann N Y Acad Sci 2007; 1114: 48–55.
Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J et al. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology 2013; 38: 1835–1842.
Glass D, Parts L, Knowles D, Aviv A, Spector TD. No correlation between childhood maltreatment and telomere length. Biol Psychiatry 2010; 68: e21.
Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry 2013; 73: 15–23.
Shalev I. Early life stress and telomere length: investigating the connection and possible mechanisms. BioEssays 2012; 34: 943–952.
Mather KA, Jorm AF, Parslow RA, Christensen H. Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci 2011; 66: 202–213.
de Jesus BB, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F et al. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med 2012; 4: 691–704.
Damjanovic AK, Yang YH, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's disease patients. J Immunol 2007; 179: 4249–4254.
Carrero J, Stenvinkel P, Fellström B, Qureshi A, Lamb K, Heimbürger O et al. Telomere attrition is associated with inflammation, low fetuin‐A levels and high mortality in prevalent haemodialysis patients. J Intern Med 2008; 263: 302–312.
Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M et al. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell 2007; 6: 639–647.
Solorio S, Murillo-Ortiz B, Hernandez-Gonzalez M, Guillen-Contreras J, Arenas-Aranda D, Solorzano-Zepeda FJ et al. Association between telomere length and C-reactive protein and the development of coronary collateral circulation in patients with coronary artery disease. Angiology 2011; 62: 467–472.
Masi S, Nightingale CM, Day IN, Guthrie P, Rumley A, Lowe GD et al. Inflammation and not cardiovascular risk factors is associated with short leukocyte telomere length in 13-to 16-year-old adolescents. Arterioscler Thromb Vasc Biol 2012; 32: 2029–2034.
Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 2006; 29: 283–289.
Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PLoS ONE 2010; 5 ((1)): e8612.
Harris SE, Martin-Ruiz C, von Zglinicki T, Starr JM, Deary IJ. Telomere length and aging biomarkers in 70-year-olds: the Lothian Birth Cohort 1936. Neurobiol Aging 2012; 331486 e 1483–1488.
Sanders JL, Fitzpatrick AL, Boudreau RM, Arnold AM, Aviv A, Kimura M et al. Leukocyte telomere length is associated with noninvasively measured age-related disease: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 2012; 67: 409–416.
Biegler KA, Anderson A, Wenzel LB, Osann K, Nelson EL. Longitudinal changes in telomere length and chronic stress response: implications for cancer prevention from a randomized biobehavioral clinical study. Cancer Prev Res 2012; 5: 1173–1182.
Ornish D, Lin J, Chan JM, Epel E, Kemp C, Weidner G et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol 2013; 14: 1112–1120.
Daubenmier J, Lin J, Blackburn E, Hecht FM, Kristeller J, Maninger N et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology 2012; 37: 917–928.
Moffitt TE and the Klaus-Grawe 2012 Think Tank. Childhood exposure to violence and lifelong health: clinical intervention science and stress biology research join forces. Dev Psychopathol (25th Anniversary Special issue) 2013; 25: 1619–1634.
Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry 2010; 197: 378–385.
Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW et al. Role of genotype in the cycle of violence in maltreated children. Science 2002; 297: 851–854.
Breslau N, Novak SP, Kessler RC. Psychiatric disorders and stages of smoking. Biol Psychiatry 2004; 55: 69–76.
McGrath M, Wong JYY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidem Bio 2007; 16: 815–819.
Kessler RC, Walters EE, Aguilar-Gaxiola S, Andrade L, Borges LG, Caraveo-Anduaga JJ et al. Cross-national Comparisons of Co-morbidities Between Substance Use Disorders and Mental Disorders. Handbook of Drug Abuse Prevention: Springer Science+Business Media LLC: New York, NY, USA, 2006, pp 447–472.
Pavanello S, Hoxha M, Dioni L, Bertazzi PA, Snenghi R, Nalesso A et al. Shortened telomeres in individuals with abuse in alcohol consumption. Int J Cancer 2011; 129: 983–992.
Yang ZY, Ye JY, Li CD, Zhou DZ, Shen Q, Wu J et al. Drug addiction is associated with leukocyte telomere length. Sci Rep 2013; 3: 1542.
Mackin P. Cardiac side effects of psychiatric drugs. Hum Psychopharmacol 2008; 23 ((Suppl 1)): 3–14.
Scott KM, Bruffaerts R, Tsang A, Ormel J, Alonso J, Angermeyer MC et al. Depression-anxiety relationships with chronic physical conditions: results from the World Mental Health Surveys. J Affect Disord 2007; 103: 113–120.
Lorant V, Deliege D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol 2003; 157: 98–112.
Robertson T, Batty GD, Der G, Fenton C, Shiels PG, Benzeval M. Is socioeconomic status associated with biological aging as measured by telomere length? Epidemiol Rev 2013; 35: 98–111.
Milne B, Byun U, Lee A. New Zealand Socio-economic Index 2006. Wellington: Statistics New Zealand, Wellington, New Zealand, 2013.
Acknowledgements
We thank the Dunedin Study members, Unit research staff, Bob Hancox, Murray Thomson and Study founder Phil Silva. This research received support from the US National Institute on Aging (AG032282) and the UK Medical Research Council (MR/K00381X). The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council. Additional support was provided by the Klaus-Grawe Foundation and the Jacobs Foundation. The study protocol was approved by the institutional ethical review boards of the participating universities. Study members gave informed consent before participating.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Shalev, I., Moffitt, T., Braithwaite, A. et al. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol Psychiatry 19, 1163–1170 (2014). https://doi.org/10.1038/mp.2013.183
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2013.183
Keywords
This article is cited by
-
Association between telomere length and neuropsychological function at 4–5 years in children from the INMA project: a cross-sectional study
European Child & Adolescent Psychiatry (2024)
-
Antidepressant medication use and prostate cancer recurrence in men with depressive disorders
Cancer Causes & Control (2022)
-
Depression, Religiosity, and Telomere Length in the Study on Stress, Spirituality, and Health (SSSH)
International Journal of Mental Health and Addiction (2022)
-
TERT rs2736100 and TERC rs16847897 genotypes moderate the association between internalizing mental disorders and accelerated telomere length attrition among HIV+ children and adolescents in Uganda
BMC Medical Genomics (2021)
-
Pediatric PTSD is characterized by age- and sex-related abnormalities in structural connectivity
Neuropsychopharmacology (2021)