Abstract
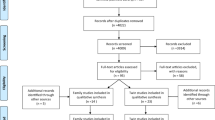
Twin studies indicate that obsessive–compulsive disorder (OCD) is strongly influenced by additive genetic factors. Yet, molecular genetic association studies have yielded inconsistent results, possibly because of differences across studies in statistical power. Meta-analysis can yield greater power. This study reports the first comprehensive meta-analysis of the relationship between OCD and all previously examined polymorphisms for which there was sufficient information in the source studies to compute odds ratios (ORs). A total of 230 polymorphisms from 113 genetic association studies were identified. A full meta-analysis was conducted for 20 polymorphisms that were examined in 5 or more data sets, and a secondary meta-analysis (limited to the computation of mean effect sizes) was conducted for 210 polymorphisms that were examined in fewer than 5 data sets. In the main meta-analysis, OCD was associated with serotonin-related polymorphisms (5-HTTLPR and HTR2A) and, in males only, with polymorphisms involved in catecholamine modulation (COMT and MAOA). Nonsignificant trends were identified for two dopamine-related polymorphisms (DAT1 and DRD3) and a glutamate-related polymorphism (rs3087879). The secondary meta-analysis identified another 18 polymorphisms with significant ORs that merit further investigation. This study demonstrates that OCD is associated with multiple genes, with most having a modest association with OCD. This suggests a polygenic model of OCD, consistent with twin studies, in which multiple genes make small, incremental contributions to the risk of developing the disorder. Future studies, with sufficient power to detect small effects, are needed to investigate the genetic basis of OCD subtypes, such as early vs late onset OCD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders,, 4th edn, text rev edn. Author: Washington, DC, 2000.
Taylor S, Jang KL, Asmundson GJG . Etiology of obsessions and compulsions: a behavioral-genetic analysis. J Abnorm Psychol 2010; 119: 672–682.
Taylor S . Etiology of obsessions and compulsions: a meta-analysis and narrative review of twin studies. Clin Psychol Rev 2011; 31: 1361–1372.
Plomin R, Defries JC, Craig IW, McGuffin P . Behavioral Genetics in the Postgenomic Era. American Psychological Association: Washington, DC, 2002.
Abramowitz JS, Taylor S, McKay D . Obsessive-compulsive disorder. Lancet 2009; 374: 491–499.
Pauls DL . The genetics of obsessive-compulsive disorder: a review. Dialogues Clin Neurosci 2010; 12: 149–163.
Borenstein M, Hedges LV, Higgins J, Rothstein H . Introduction to Meta-Analysis. Wiley: Chichester, 2009.
Taylor S . Early versus late onset obsessive-compulsive disorder: evidence for distinct subtypes. Clin Psychol Rev 2011; 31: 1083–1100.
Azzam A, Mathews CA . Meta-analysis of the association between the catecholamine-O-methyl-transferase gene and obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet 2003; 123: 64–69.
Pooley EC, Fineberg N, Harrison PJ . The met158 allele of catechol-O-methyltransferase (COMT) is associated with obsessive-compulsive disorder in men: case-control study and meta-analysis. Mol Psychiatry 2007; 12: 556–561.
Schaid DJ . Transmission disequilibrium, family controls, and great expectations. Am J Hum Genet 1998; 63: 935–941.
Lewis CM . Genetic association studies: design, analysis and interpretation. Brief Bioinform 2002; 3: 146–153.
Lin PY . Meta-analysis of the association of serotonin transporter gene polymorphism with obsessive–compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31: 683–689.
Bloch MH, Landeros-Weisenberger A, Sen S, Dombrowski P, Kelmendi B, Corci V et al. Association of the serotonin transporter polymorphism and obsessive-compulsive disorder: systematic review. Am J Med Genet B Neuropsychiatr Genet 2008; 147: 850–858.
Voyiaziakis E, Evgrafov O, Li D, Yoon HJ, Tabares P, Samuels J et al. Association of SLC6A4 variants with obsessive-compulsive disorder in a large multicenter US family study. Mol Psychiatry 2011; 16: 108–120.
Wendland JR KM, Cromer KR, Murphy DL . A large case-control study of common functional SLC6A4 and BDNF variants in obsessive-compulsive disorder. Neuropsychopharmacology 2007; 32: 2543–2551.
Lefebvre C, Manheimer E, Glanville J . Searching for studies. In: Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Wiley: New York, 2008 pp 146–153.
Borenstein M, Hedges LV, Higgins J, Rothstein H . Comprehensive Meta-Analysis, 2.2050 edn. Biostat: Englewood, NJ, 2009.
Perneger TV . What′s wrong with Bonferroni adjustments. BMJ 1998; 316: 1236–1238.
Rothman K . No adjustments are needed for multiple comparisons. Epidemiology 1990; 1: 43–46.
Lieberman MD, Cunningham WA . Type I and type II error concerns in fMRI research: re-balancing the scale. SCAN 2009; 4: 423–428.
Gravetter FJ, Wallnau LB . Essentials of Statistics for the Behavioral Sciences, 7 edn. Wadsworth: Belmont, CA, 2011.
Smith LF, Gratz ZS, Bousquet SG . The Art and Practice of Statistics. Wadsworth: Belmont, CA, 2009.
Gordi T, Khamis H . Simple solution to a common statistical problem: interpreting multiple tests. Clin Ther 2004; 26: 780–786.
Holm S . A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6: 65–70.
Egger M, Smith G, Schneider M, Minder C . Bias in meta-analysis detected by a simple graphical test. BMJ 1997; 315: 629–634.
Dickel DE, Veenstra-VanderWeele J, Bivens NC, Wu X, Fischer DJ, Van Etten-Lee M et al. Association studies of serotonin system candidate genes in early-onset obsessive-compulsive disorder. Biol Psychiatry 2007; 61: 322–329.
Cohen J . Statistical Power Analyses for the Behavioral Sciences, 2nd edn. Erlbaum: Hillsdale, NJ, 1988.
Karayiorgou M, Sobin C, Blundell ML, Galke BL, Malinova L, Goldberg P et al. Family-based association studies support a sexually dimorphic effect of COMT and MAOA on genetic susceptibility to obsessive-compulsive disorder. Biol Psychiatry 1999; 45: 1178–1189.
Zintzaras E, Lau J . Trends in meta-analysis of genetic association studies. J Hum Genet 2008; 53: 1–9.
Rajender G, Bhatia MS, Kanwal K, Malhotra S, Singh TB, Chaudhary D . Study of neurocognitive endophenotypes in drug-naïve obsessive-compulsive disorder patients, their first-degree relatives and health controls. Acta Psychiatr Scand 2011; 124: 152–161.
Gatacòs M, Costas J, de Cid R, Bayés M, González JR, Baca-García E et al. Identification of new putative susceptibility genes for several psychiatric disorders by association analysis of regulatory and non-synonymous SNPs of 306 genes involved in neurotransmission and neurodevelopment. Am J Med Genet B Neuropsychiatr Genet 2009; 150: 808–816.
Samuels J, Wang Y, Riddle MA, Greenberg BD, Fyer AJ, McCracken JT et al. Comprehensive family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet 2011; 156: 472–477.
da Rocha FF, Marco LA, Romano-Silva MA, Corrêa H . Obsessive-compulsive disorder and 5-HTTLPR. Rev Bras Psiquiatr 2009; 31: 281–292.
Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet 2006; 78: 815–826.
Tibrewal P, Kumar HB, Shubha GN, Subhashree D, Purushottam M, Thennarasu K et al. Association of serotonin transporter gene polymorphisms with obsessive-compulsive disorder (OCD) in a south Indian population. Indian J Med Res 2010; 132: 690–695.
Denys D, van Nieuwerburgh F, Deforce D, Westenberg HGM . Association between serotonergic candidate genes and specific phenotypes of obsessive compulsive disorder. J Affect Disord 2006; 91: 39–44.
Enoch MA, Kaye WH, Rotondo A, Greenberg BD, Murphy DL, Goldman D . 5-HT2A promoter polymorphism −1438G/A, anorexia nervosa, and obsessive-compulsive disorder. Lancet 1998; 351: 1785–1786.
Enoch MA, Greenberg BD, Murphy DL, Goldman D . Sexually dimorphic relationship of a 5-HT2A promoter polymorphism with obsessive-compulsive disorder. Biol Psychiatry 2001; 49: 385–388.
Frisch A, Michaelovsky E, Rockah R, Amir I, Hermesh H, Laor N et al. Association between obsessive-compulsive disorder and polymorphisms of genes encoding components of the serotonergic and dopaminergic pathways. Eur Neuropsychopharmacol 2000; 10: 205–209.
Gruenblatt E, Romanos M, Renner T, Walitza S . Copy number variations in children and adolescents with early onset obsessive-compulsive disorder. Eur Child Adolesc Psychiatry 2011; 20 (Suppl 1): S44–S45.
Hemmings SMJ Investigating the molecular aetiology of obsessive-compulsive disorder (OCD) and clinically-defined subsets of OCD. Ph.D. thesis, University of Stellenbosch, South Africa, 2006.
Jung HR, Cho JY, Chung JY, Kin JR, Yu KS, Jang IJ et al. No associations between 5-HTT, 5-HT2A gene polymorphisms and obsessive-compulsive disorder in a Korean population. Psychiatry Invest 2006; 3: 78–86.
Liu W, Zhao N, Xiong J, Shi M, Hu J . Associated analysis of serotonin and catecholamine system candidate genes in obsessive–compulsive disorder in the Chinese population. Psychiatry Res 2011; 188: 170–172.
Meira-Lima I, Shavitt RG, Miguita K, Ikenage E, Miguel EC, Vallada H . Association analysis of the catechol-o-methyltransferase (COMT), serotonin transporter (5-HTT) and serotonin 2A receptor (5HT2A) gene polymorphisms with obsessive-compulsive disorder. Genes Brain Behav 2004; 3: 75–79.
Nicolini H, Cruz C, Camarena B, Orozco B, Kennedy JL, King N et al. DRD2, DRD3 and 5HT2A receptor genes polymorphisms in obsessive-compulsive disorder. Mol Psychiatry 1996; 1: 461–465.
Saiz PA, Garcia-Portilla MP, Arango C, Morales B, Bascaran MT, Martinez-Barrondo S et al. Association study between obsessive-compulsive disorder and serotonergic candidate genes. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32: 765–770.
Tot S, Erdal EM, Yazici K, Yazici AE, Metin O . T102C and −1438 G/A polymorphisms of the 5-HT2A receptor gene in Turkish patients with obsessive–compulsive disorder. Eur Psychiatry 2003; 18: 249–254.
Walitza S, Wewetzer C, Warnke A, Gerlach M, Geller F, Gerber G et al. 5-HT2A promoter polymorphism −1438G/A in children and adolescents with obsessive-compulsive disorders. Mol Psychiatry 2002; 7: 1054–1057.
Alsobrook JP, Zohar AH, Leboyer M, Chabane N, Ebstein RP, Pauls DL . Association between the COMT locus and obsessive-compulsive disorder in females but not males. Am J Med Genet B Neuropsychiatr Genet 2002; 114: 116–120.
Karayiorgou M, Altemusm M, Galke BL, Goldman D, Murphy DL, Ott J et al. Genotype determining low catechol-O-methyltransferase activity as a risk factor for obsessive-compulsive disorder. Proc Natl Acad Sci USA 1997; 94: 4572–4575.
Katerberg H, Cath DC, Denys DA, Heutink P, Polman A, van Nieuwerburgh FC et al. The role of the COMT Val158Met polymorphism in the phenotypic expression of obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet 2010; 153: 167–176.
Poyurovsky M, Michaelovsky E, Frisch A, Knoll G, Amir I, Buniak F et al. COMT Val158Met polymorphism in schizophrenia with obsessive-compulsive disorder: a case-control study. Neurosci Lett 2005; 389: 21–24.
Schindler KM, Richter MA, Kennedy JL, Pato MT, Pato CN . Association between homozygosity at the COMT gene locus and obsessive compulsive disorder. Am J Med Genet B Neuropsychiatr Genet 2000; 96: 721–724.
Walitza S, Scherag A, Renner TJ, Hinney A, Remschmidt H, Herpertz-Dahlmann B et al. Transmission disequilibrium studies in early onset of obsessive-compulsive disorder for polymorphisms in genes of the dopaminergic system. J Neural Transm 2008; 115: 1071–1078.
Camarena B, Rinetti G, Cruz C, Gomez A, de la Fuente JR, Nicolini H . Additional evidence that genetic variation of MAO-A gene supports a gender subtype in obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet 2001; 105: 279–282.
Lochner C, Hemmings SM, Kinnear CJ, Moolman-Smook JC, Corfield VA, Knowles JA et al. Gender in obsessive-compulsive disorder: clinical and genetic findings. Eur Neuropsychopharmacol 2004; 14: 105–113.
Acknowledgements
I am grateful to the following investigators for providing unpublished details of their research, which facilitated the completion of this study: Beatriz Camarena, Sîan MJ Hemmings, Humberto Nicolini, Dan J Stein, Jeremy M Veenstra-VanderWeele and Susanne Walitza.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Taylor, S. Molecular genetics of obsessive–compulsive disorder: a comprehensive meta-analysis of genetic association studies. Mol Psychiatry 18, 799–805 (2013). https://doi.org/10.1038/mp.2012.76
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2012.76
Keywords
This article is cited by
-
Autoantibodies in patients with obsessive-compulsive disorder: a systematic review
Translational Psychiatry (2023)
-
Perinatal Obsessive–Compulsive Disorder: Epidemiology, Phenomenology, Etiology, and Treatment
Current Psychiatry Reports (2022)
-
Transcriptome alterations are enriched for synapse-associated genes in the striatum of subjects with obsessive-compulsive disorder
Translational Psychiatry (2021)
-
A dimensional perspective on the genetics of obsessive-compulsive disorder
Translational Psychiatry (2021)
-
Risk factors for obsessive–compulsive symptoms. Follow-up of a community-based youth cohort
European Child & Adolescent Psychiatry (2021)