Abstract
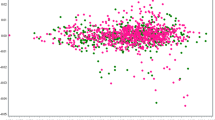
We report the first genome-wide association study in 1000 bipolar I patients and 1000 controls, with a replication of the top hits in another 409 cases and 1000 controls in the Han Chinese population. Four regions with most strongly associated single-nucleotide polymorphisms (SNPs) were detected, of which three were not found in previous GWA studies in the Caucasian populations. Among them, SNPs close to specificity protein 8 (SP8) and ST8 α-N-acetyl- neuraminide α-2,8-sialyltransferase (ST8SIA2) are associated with Bipolar I, with P-values of 4.87 × 10−7 (rs2709736) and 6.05 × 10−6 (rs8040009), respectively. We have also identified SNPs in potassium channel tetramerization domain containing 12 gene (KCTD12) (rs2073831, P=9.74 × 10−6) and in CACNB2 (Calcium channel, voltage-dependent, β-2 subunit) gene (rs11013860, P=5.15 × 10−5), One SNP nearby the rs1938526 SNP of ANK3 gene and another SNP nearby the SNP rs11720452 in chromosome 3 reported in previous GWA studies also showed suggestive association in this study (P=6.55 × 10−5 and P=1.48 × 10−5, respectively). This may suggest that there are common and population-specific susceptibility genes for bipolar I disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Smoller JW, Finn CT . Family, twin, and adoption studies of bipolar disorder. Am J Med Genet C Semin Med Genet 2003; 123C: 48–58.
The Welcome Trust Case Control Consortium. Genome-wide association study of 14 000 cases of seven common diseases and 3000 shared controls. Nature 2007; 447: 661–678.
Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry 2008; 13: 197–207.
Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008; 40: 1056–1058.
Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K et al. Whole-genome association study of bipolar disorder. Mol Psychiatry 2008; 13: 558–569.
Brotman AW, Farhadi AM, Gelenberg AJ . Verapamil treatment of acute mania. J Clin Psychiatry 1986; 47: 136–138.
Goodnick PJ . The use of nimodipine in the treatment of mood disorders. Bipolar Disord 2000; 2: 165–173.
Hough C, Lu SJ, Davis CL, Chuang DM, Post RM . Elevated basal and thapsigargin-stimulated intracellular calcium of platelets and lymphocytes from bipolar affective disorder patients measured by a fluorometric microassay. Biol Psychiatry 1999; 46: 247–255.
Moller HJ . Bipolar disorder and schizophrenia: distinct illnesses or a continuum? J Clin Psychiatry 2003; 64 (Suppl 6): 23–27; discussion 28.
Tsuang MT, Winokur G, Crowe RR . Morbidity risks of schizophrenia and affective disorders among first degree relatives of patients with schizophrenia, mania, depression and surgical conditions. Br J Psychiatry 1980; 137: 497–504.
Valles V, Van Os J, Guillamat R, Gutierrez B, Campillo M, Gento P et al. Increased morbid risk for schizophrenia in families of in-patients with bipolar illness. Schizophr Res 2000; 42: 83–90.
Cardno AG, Rijsdijk FV, Sham PC, Murray RM, McGuffin P . A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry 2002; 159: 539–545.
McGuffin P, Reveley A, Holland A . Identical triplets: non-identical psychosis? Br J Psychiatry 1982; 140: 1–6.
Badner JA, Gershon ES . Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry 2002; 7: 405–411.
Berrettini W . Evidence for shared susceptibility in bipolar disorder and schizophrenia. Am J Med Genet C Semin Med Genet 2003; 123C: 59–64.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. American Psychiatric Association: Washington, DC, 1994.
Cheng ATA, Tien AY, Chang CJ, Brugha TS, Cooper JE, Lee CS et al. Cross-cultural implementation of a Chinese version of the Schedules for Clinical Assessment in Neuropsychiatry (SCAN) in Taiwan. Br J Psychiatry 2001; 178: 567–572.
Lee CS, Liu CY, Chang CJ, Chang JC, Cheng TA, Yu WY et al. Inter-rater Reliability of the Chinese Version of the Schedules for Clinical Assessment in Neuropsychiatry (SCAN) in Taiwan. Taiwan J Psychiatry 2002; 16: 35–45.
World Health Organization. SCAN I-Shell. Computer assisted personal interviewing application for the Schedules for Clinical Assessment in Neuropsychiatry version 2.1 and diagnostic algorithms for WHO ICD-10 Chapter V DCR and for American Psychiatric Association Diagnostic and Statistical Manual. Version 1.0.4.6. World Health Organization: Geneva, 2005.
Pan WH, Fann CSJ, Wu JY, Hung YT, Ho MS, Tai TH et al. Han Chinese cell and genome bank in Taiwan: purpose, design and ethical considerations. Hum Hered 2006; 61: 27–30.
Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA . Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet 2002; 70: 425–434.
Smith EN, Bloss CS, Badner JA, Barrett T, Belmonte PL, Berrettini W et al. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry 2009; 14: 755–763.
Regier DA, Kaelber CT, Roper MT, Rae DS, Sartorius N . The ICD-10 clinical field trial for mental and behavioral disorders: results in Canada and the United States. Am J Psychiatry 1994; 151: 1340–1350.
Harkavy-Friedman JM, Keilp JG, Grunebaum MF, Sher L, Printz D, Burke AK ; et al. Are BPI and BPII suicide attempters distinct neuropsychologically? J Affect Disord 2006; 94: 255–259.
Fossion P, Staner L, Dramaix M, Kempenaers C, Kerkhofs M, Hubain P et al. Does sleep EEG data distinguish between UP, BPI or BPII major depressions? An age and gender controlled study. J Affect Disord 1998; 49: 181–187.
Maier W, Lichtermann D, Minges J, Hallmayer J, Heun R, Benkert O et al. Continuity and discontinuity of affective disorders and schizophrenia. Arch Gen Psychiatry 1993; 50: 871–883.
Zembrzycki A, Griesel G, Stoykova A, Mansouri A . Genetic interplay between the transcription factors Sp8 and Emx2 in the patterning of the forebrain. Neural Dev 2007; 2: 8.
O’Leary DD, Sahara S . Genetic regulation of arealization of the neocortex. Curr Opin Neurobiol 2008; 18: 90–100.
Vazza G, Bertolin C, Scudellaro E, Vettori A, Boaretto F, Rampinelli S et al. Genome-wide scan supports the existence of a susceptibility locus for schizophrenia and bipolar disorder on chromosome 15q26. Mol Psychiatry 2007; 12: 87–93.
Angata K, Suzuki M, McAuliffe J, Ding Y, Hindsgaul O, Fukuda M . Differential biosynthesis of polysialic acid on neural cell adhesion molecule (NCAM) and oligosaccharide acceptors by three distinct alpha 2,8-sialyltransferases, ST8Sia IV (PST), ST8Sia II (STX), and ST8Sia III. J Biol Chem 2000; 275: 18594–18601.
Arai M, Itokawa M, Yamada K, Toyota T, Arai M, Haga S et al. Association of neural cell adhesion molecule 1 gene polymorphisms with bipolar affective disorder in Japanese individuals. Biol Psychiatry 2004; 55: 804–810.
Atz ME, Rollins B, Vawter MP . NCAM1 association study of bipolar disorder and schizophrenia: polymorphisms and alternatively spliced isoforms lead to similarities and differences. Psychiatr Genet 2007; 17: 55–67.
Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C . A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res 2004; 71: 405–416.
Resendes BL, Kuo SF, Robertson NG, Giersch AB, Honrubia D, Ohara O et al. Isolation from cochlea of a novel human intronless gene with predominant fetal expression. J Assoc Res Otolaryngol 2004; 5: 185–202.
Goldstein I, Lerer E, Laiba E, Mallet J, Mujaheed M, Laurent C et al. Association between sodium- and potassium-activated adenosine triphosphatase alpha isoforms and bipolar disorders. Biol Psychiatry 2009; 65: 985–991.
Kato T . Molecular neurobiology of bipolar disorder: a disease of ‘mood-stabilizing neurons’? Trends Neurosci 2008; 31: 495–503.
Acknowledgements
This study was supported by Academia Sinica Genomic Medicine Multicenter Study and National Research Program for Genomic Medicine, National Science Council, Taiwan (National Clinical Core, NSC97-3112-B-001-014 and National Genotyping Center, NSC97-3112-B-001-015). We thank other members of the Taiwan Bipolar Consortium (Tsry Huey Mental Hospital, Pingtong, Taiwan, Jung-Kwang Wen, MD and Ching-Kuan Wu, MD; Beitou Hospital, Taipei, Taiwan, Sy-Ueng Luu, MD; Ju Shan Hospital, Taoyuan, Taiwan, Shi-Chin Guo, MD; Ping An Hospital, Pingtong, Taiwan, Wen-Hsiang Huang, MD) for their contributions in the recruitment of bipolar I patients and the families who devoted their time and effort to the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Lee, M., Chen, C., Lee, C. et al. Genome-wide association study of bipolar I disorder in the Han Chinese population. Mol Psychiatry 16, 548–556 (2011). https://doi.org/10.1038/mp.2010.43
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2010.43
Keywords
This article is cited by
-
Functional modular networks identify the pivotal genes associated with morphine addiction and potential drug therapies
BMC Anesthesiology (2023)
-
Compromised mammillary body connectivity and psychotic symptoms in mice with di- and mesencephalic ablation of ST8SIA2
Translational Psychiatry (2022)
-
Combined cellomics and proteomics analysis reveals shared neuronal morphology and molecular pathway phenotypes for multiple schizophrenia risk genes
Molecular Psychiatry (2021)
-
Bipolar multiplex families have an increased burden of common risk variants for psychiatric disorders
Molecular Psychiatry (2021)
-
Amygdala GluN2B-NMDAR dysfunction is critical in abnormal aggression of neurodevelopmental origin induced by St8sia2 deficiency
Molecular Psychiatry (2020)