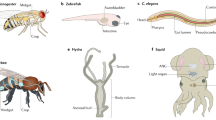
Abstract
While the research community has accepted the value of rodent models as informative research platforms, there is less awareness of the utility of other small vertebrate and invertebrate animal models. Neuroscience is increasingly turning to smaller, non-rodent models to understand mechanisms related to neuropsychiatric disorders. Although they can never replace clinical research, there is much to be learnt from ‘small brains’. In particular, these species can offer flexible genetic ‘tool kits’ that can be used to explore the expression and function of candidate genes in different brain regions. Very small animals also offer efficiencies with respect to high-throughput screening programs. This review provides a concise overview of the utility of models based on worm, fruit fly, honeybee and zebrafish. Although these species may have small brains, they offer the neuropsychiatric research community opportunities to explore some of the most important research questions in our field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Tallman JF . Neuropsychopharmacology at the new millennium: new industry directions. Neuropsychopharmacology 1999; 20: 99–105.
Miczek KA, de Wit H . Challenges for translational psychopharmacology research—some basic principles. Psychopharmacology (Berl) 2008; 199: 291–301.
Marsden CA, Rex A . Transgenics and psychopharmacology—introduction. Rev Neurosci 2000; 11: 1–2.
Cryan JF, Holmes A . The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 2005; 4: 775–790.
German DC, Eisch AJ . Mouse models of Alzheimer's disease: insight into treatment. Rev Neurosci 2004; 15: 353–369.
Tecott LH . The genes and brains of mice and men. Am J Psychiatry 2003; 160: 646–656.
Arguello PA, Gogos JA . Modeling madness in mice: one piece at a time. Neuron 2006; 52: 179–196.
Crawley JN . Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol 2007; 17: 448–459.
White JG, Southgate E, Thomson JN, Brenner S . The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 1986; 314: 1–340.
Chatterjee N, Sinha S . Understanding the mind of a worm: hierarchical network structure underlying nervous system function in C. elegans. Progress in Brain Research 2007; Vol 168: pp 145–153.
Kendler KS, Greenspan RJ . The nature of genetic influences on behavior: lessons from ‘simpler’ organisms. Am J Psychiatry 2006; 163: 1683–1694.
Sawamura N, Ando T, Maruyama Y, Fujimuro M, Mochizuki H, Honjo K et al. Nuclear DISC1 regulates CRE-mediated gene transcription and sleep homeostasis in the fruit fly. Mol Psychiatry 2008; 13: 1138–1148, 1069.
Sawamura N, Ishida N, Tomoda T, Hai T, Furukubo-Tokunaga K, Sawa A . The fruitfly Drosophila melanogaster: a promising model to explore molecular psychiatry. Mol Psychiatry 2008; 13: 1069.
Sulston JE, Schierenberg E, White JG, Thomson JN . The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 1983; 100: 64–119.
Hall DH, Russell RL . The posterior nervous system of the nematode Caenorhabditis elegans: serial reconstruction of identified neurons and complete pattern of synaptic interactions. J Neurosci 1991; 11: 1–22.
Takahashi K, Yoshina S, Masashi M, Ito W, Inoue T, Shiwaku H et al. Nematode homologue of PQBP1, a mental retardation causative gene, is involved in lipid metabolism. PLoS ONE 2009; 4: e4104.
Grigorenko AP, Moliaka YK, Soto MC, Mello CC, Rogaev EI . The Caenorhabditis elegans IMPAS gene, imp-2, is essential for development and is functionally distinct from related presenilins. Proc Natl Acad Sci USA 2004; 101: 14955–14960.
Bloom L, Horvitz HR . The Caenorhabditis elegans gene unc-76 and its human homologs define a new gene family involved in axonal outgrowth and fasciculation. Proc Natl Acad Sci USA 1997; 94: 3414–3419.
Miyoshi K, Honda A, Baba K, Taniguchi M, Oono K, Fujita T et al. Disrupted-In-Schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol Psychiatry 2003; 8: 685–694.
Bord L, Wheeler J, Paek M, Saleh M, Lyons-Warren A, Ross CA et al. Primate disrupted-in-schizophrenia-1 (DISC1): high divergence of a gene for major mental illnesses in recent evolutionary history. Neurosci Res 2006; 56: 286–293.
Jin Y, Jorgensen E, Hartwieg E, Horvitz HR . The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J Neurosci 1999; 19: 539–548.
Nass R, Miller DM, Blakely RD . C. elegans: a novel pharmacogenetic model to study Parkinson's disease. Parkinsonism Relat Disord 2001; 7: 185–191.
Sze JY, Victor M, Loer C, Shi Y, Ruvkun G . Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 2000; 403: 560–564.
Rugarli EI, Di Schiavi E, Hilliard MA, Arbucci S, Ghezzi C, Facciolli A et al. The Kallmann syndrome gene homolog in C. elegans is involved in epidermal morphogenesis and neurite branching. Development 2002; 129: 1283–1294.
de Bono M, Maricq AV . Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci 2005; 28: 451–501.
Feng Z, Li W, Ward A, Piggott BJ, Larkspur ER, Sternberg PW et al. A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell 2006; 127: 621–633.
Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A et al. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell 2003; 115: 655–666.
Giles AC, Rose JK, Rankin CH . Investigations of learning and memory in Caenorhabditis elegans. Int Rev Neurobiol 2006; 69: 37–71.
Donohoe DR, Phan T, Weeks K, Aamodt EJ, Dwyer DS . Antipsychotic drugs up-regulate tryptophan hydroxylase in ADF neurons of Caenorhabditis elegans: role of calcium-calmodulin-dependent protein kinase II and transient receptor potential vanilloid channel. J Neurosci Res 2008; 86: 2553–2563.
Raizen DM, Zimmerman JE, Maycock MH, Ta UD, You YJ, Sundaram MV et al. Lethargus is a Caenorhabditis elegans sleep-like state. Nature 2008; 451: 569–572.
Suo S, Ishiura S, Van Tol HH . Dopamine receptors in C. elegans. Eur J Pharmacol 2004; 500: 159–166.
Wolf FW, Heberlein U . Invertebrate models of drug abuse. J Neurobiol 2003; 54: 161–178.
Zubenko GS, Jones ML, Estevez AO, Hughes III HB, Estevez M . Identification of a CREB-dependent serotonergic pathway and neuronal circuit regulating foraging behavior in Caenorhabditis elegans: a useful model for mental disorders and their treatments? Am J Med Genet B Neuropsychiatr Genet 2009; 150B: 12–23.
Benzer S . Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc Natl Acad Sci USA 1967; 58: 1112–1119.
Tully T, Quinn WG . Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A 1985; 157: 263–277.
Davis RL . Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci 2005; 28: 275–302.
Hardin PE . The circadian timekeeping system of Drosophila. Curr Biol 2005; 15: R714–R722.
Greenspan RJ, Ferveur JF . Courtship in Drosophila. Annu Rev Genet 2000; 34: 205–232.
Vosshall LB . Into the mind of a fly. Nature 2007; 450: 193–197.
van Swinderen B . Attention-like processes in Drosophila require short-term memory genes. Science 2007; 315: 1590–1593.
Shaw PJ, Cirelli C, Greenspan RJ, Tononi G . Correlates of sleep and waking in Drosophila melanogaster. Science 2000; 287: 1834–1837.
Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S . dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci USA 1976; 73: 1684–1688.
Davis RL, Cherry J, Dauwalder B, Han PL, Skoulakis E . The cyclic AMP system and Drosophila learning. Mol Cell Biochem 1995; 149–150: 271–278.
Davis RL, Dauwalder B . The Drosophila dunce locus: learning and memory genes in the fly. Trends Genet 1991; 7: 224–229.
Anderton BH . Alzheimer's disease: clues from flies and worms. Curr Biol 1999; 9: R106–R109.
Gotz J, Streffer JR, David D, Schild A, Hoerndli F, Pennanen L et al. Transgenic animal models of Alzheimer's disease and related disorders: histopathology, behavior and therapy. Mol Psychiatry 2004; 9: 664–683.
Simon AF, Liang DT, Krantz DE . Differential decline in behavioral performance of Drosophila melanogaster with age. Mech Ageing Dev 2006; 127: 647–651.
Apostol BL, Kazantsev A, Raffioni S, Illes K, Pallos J, Bodai L et al. A cell-based assay for aggregation inhibitors as therapeutics of polyglutamine-repeat disease and validation in Drosophila. Proc Natl Acad Sci USA 2003; 100: 5950–5955.
Cooper RL, Neckameyer WS . Dopaminergic modulation of motor neuron activity and neuromuscular function in Drosophila melanogaster. Comp Biochem Physiol B Biochem Mol Biol 1999; 122: 199–210.
Steffan JS, Bodai L, Pallos J, Poelman M, McCampbell A, Apostol BL et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 2001; 413: 739–743.
Li J, Ashley J, Budnik V, Bhat MA . Crucial role of Drosophila neurexin in proper active zone apposition to postsynaptic densities, synaptic growth, and synaptic transmission. Neuron 2007; 55: 741–755.
Zeng X, Sun M, Liu L, Chen F, Wei L, Xie W . Neurexin-1 is required for synapse formation and larvae associative learning in Drosophila. FEBS Lett 2007; 581: 2509–2516.
Bolduc FV, Bell K, Cox H, Broadie KS, Tully T . Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat Neurosci 2008; 11: 1143–1145.
De Luca V, Muglia P, Jain U, Basile VS, Sokolowski MB, Kennedy JL . A drosophila model for attention deficit hyperactivity disorder (ADHD): no evidence of association with PRKG1 gene. Neuromolecular Med 2002; 2: 281–287.
Guarnieri DJ, Heberlein U . Drosophila melanogaster, a genetic model system for alcohol research. Int Rev Neurobiol 2003; 54: 199–228.
Scholz H . Intoxicated fly brains: neurons mediating ethanol-induced behaviors. J Neurogenet 2009; 23: 111–119.
Corl AB, Berger KH, Ophir-Shohat G, Gesch J, Simms JA, Bartlett SE et al. Happyhour, a Ste20 family kinase, implicates EGFR signaling in ethanol-induced behaviors. Cell 2009; 137: 949–960.
Dierick HA . A method for quantifying aggression in male Drosophila melanogaster. Nat Protoc 2007; 2: 2712–2718.
Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA, Hardie SL et al. Octopamine in male aggression of Drosophila. Curr Biol 2008; 18: 159–167.
van Swinderen B, Greenspan RJ . Salience modulates 20–30 Hz brain activity in Drosophila. Nat Neurosci 2003; 6: 579–586.
Seugnet L, Suzuki Y, Stidd R, Shaw PJ . Aversive phototaxic suppression: evaluation of a short-term memory assay in Drosophila melanogaster. Genes Brain Behav 2009; 8: 377–389.
Sarkar S, Krishna G, Imarisio S, Saiki S, O'Kane CJ, Rubinsztein DC . A rational mechanism for combination treatment of Huntington's disease using lithium and rapamycin. Human Mol Genet 2008; 17: 170–178.
Dokucu ME, Yu L, Taghert PH . Lithium- and valproate-induced alterations in circadian locomotor behavior in Drosophila. Neuropsychopharmacology 2005; 30: 2216–2224.
Gilestro GF, Tononi G, Cirelli C . Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science 2009; 324: 109–112.
van Swinderen B, Flores KA . Attention-like processes underlying optomotor performance in a Drosophila choice maze. J Neurobiol 2006; 67: 129–145.
Swinderen B . The remote roots of consciousness in fruit-fly selective attention? Bioessays 2005; 27: 321–330.
Menzel R, Giurfa M . Cognition by a mini brain. Nature 1999; 400: 718–719.
Gould JL, Gould CG . The Honeybee. Scientific American Library: New York, 1995.
von Frisch K . The Dance Language and Orientation of Bees. Harvard Univ. Press.: London, 1993.
Reinhard J, Srinivasan MV, Zhang SW . Scent-triggered navigation in honeybees. Nature 2004; 427: 411.
Giurfa M . Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J Comparative Physiol 2007; 193: 801–824.
Letzkus P, Ribi WA, Wood JT, Zhu H, Zhang SW, Srinivasan MV . Lateralization of olfaction in the honeybee Apis mellifera. Curr Biol 2006; 16: 1471–1476.
Rogers LJ, Vallortigara G . From antenna to antenna: lateral shift of olfactory memory recall by honeybees. PLoS One 2008; 3: e2340.
Rujescu D, Ingason A, Cichon S, Pietilainen OP, Barnes MR, Toulopoulou T et al. Disruption of the neurexin 1 gene is associated with schizophrenia. Human Mol Genet 2009; 18: 988–996.
Chubykin AA, Liu X, Comoletti D, Tsigelny I, Taylor P, Sudhof TC . Dissection of synapse induction by neuroligins: effect of a neuroligin mutation associated with autism. J Biol Chem 2005; 280: 22365–22374.
Biswas S, Russell RJ, Jackson CJ, Vidovic M, Ganeshina O, Oakeshott JG et al. Bridging the synaptic gap: neuroligins and neurexin I in Apis mellifera. PLoS ONE 2008; 3: e3542.
Weinstock GM, Robinson GE, Gibbs RA, Worley KC, Evans JD, Maleszka R . Insights into social insects from the genome of the honeybee Apis mellifera. Nature 2006; 443: 931–949.
Baier H, Scott EK . Genetic and optical targeting of neural circuits and behavior-zebrafish in the spotlight. Curr Opin Neurobiol 2009; 19: 553–560.
Baier H . Zebrafish on the move: towards a behavior-genetic analysis of vertebrate vision. Curr Opin Neurobiol 2000; 10: 451–455.
McLean DL, Fetcho JR . Using imaging and genetics in zebrafish to study developing spinal circuits in vivo. Dev Neurobiol 2008; 68: 817–834.
Debruyne J, Hurd MW, Gutiérrez L, Kaneko M, Tan Y, Wells DE et al. Isolation and phenogenetics of a novel circadian rhythm mutant in zebrafish. J Neurogenet 2004; 18: 403–428.
Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF . Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci 2006; 26: 13400–13410.
Bretaud S, Li Q, Lockwood BL, Kobayashi K, Lin E, Guo S . A choice behavior for morphine reveals experience-dependent drug preference and underlying neural substrates in developing larval zebrafish. Neuroscience 2007; 146: 1109–1116.
Darland T, Dowling JE . Behavioral screening for cocaine sensitivity in Mutagenized Zebrafish. Proc Natl Acad Sci USA 2001; 98: 11691–11696.
Gerlai R, Lahav M, Guo S, Rosenthal A . Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behavior 2000; 67: 773–782.
Kily LJM, Cowe YCM, Hussain O, Patel S, McElwaine S, Cotter FE et al. Gene expression changes in a zebrafish model of drug dependency suggest conservation of neuro-adaptation pathways. J Exp Biol 2008; 211: 1623–1634.
Ninkovic J, Bally-Cuif L . The zebrafish as a model system for assessing the reinforcing properties of drugs of abuse. Methods 2006; 39: 262–274.
Best JD, Berghmans S, Hunt JJFG, Clarke SC, Fleming A, Goldsmith P et al. Non-associative learning in larval zebrafish. Neuropsychopharmacology 2008; 33: 1206–1215.
Best JD, Alderton WK . Zebrafish: an in vivo model for the study of neurological diseases. Neuropsychiatric Disease and Treatment 2008; 4: 567–576.
Guo S . Linking genes to brain, behavior and neurological diseases: what can we learn from zebrafish? Genes, Brain and Behavior 2004; 3: 63–74.
Schweitzer J, Driever W . Development of the dopamine systems in zebrafish. Adv Exp Med Biol 2009; 651: 1–14.
Ekker SC, Larson JD . Morphant technology in model developmental systems. Genesis 2001; 30: 89–93.
Elliott DA, Brand AH . The GAL4 system: a versatile system for the expression of genes. Methods Mol Biol (Clifton, NJ) 2008; 420: 79–95.
Lichtman JW, Smith SJ . Seeing circuits assemble. Neuron 2008; 60: 441–448.
Arrenberg AB, Del Bene F, Baier H . Optical control of zebrafish behavior with halorhodopsin. Proc Natl Acad Sci USA 2009; 106: 17968–17973.
Borue X, Cooper S, Hirsh J, Condron B, Venton BJ . Quantitative evaluation of serotonin release and clearance in Drosophila. J Neurosci Methods 2009; 179: 300–308.
Wyart C, Del Bene F, Warp E, Scott EK, Trauner D, Baier H et al. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature 2009; 461: 407–410.
Olsen SR, Wilson RI . Cracking neural circuits in a tiny brain: new approaches for understanding the neural circuitry of Drosophila. Trends Neurosci 2008; 31: 512–520.
Wu Y, Bolduc FV, Bell K, Tully T, Fang Y, Sehgal A et al. A Drosophila model for Angelman syndrome. Proc Natl Acad Sci USA 2008; 105: 12399–12404.
Schnabel J . Neuroscience: standard model. Nature 2008; 454: 682–685.
van der Staay FJ . Animal models of behavioral dysfunctions: basic concepts and classifications, and an evaluation strategy. Brain Res Rev 2006; 52: 131–159.
Meyer U, Feldon J . Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol 2009; e-pub ahead of print.
van der Staay FJ, Arndt SS, Nordquist RE . Evaluation of animal models of neurobehavioral disorders. Behav Brain Funct 2009; 5: 11.
Kandel ER . In Search of Memory: The Emergence of a New Science of Mind Norton. New York, 2006.
Sattelle DB, Jones AK, Buckingham SD . Insect genomes: challenges and opportunities for neuroscience. Invert Neurosci 2007; 7: 133–136.
McGrath JJ, Richards LJ . Why schizophrenia epidemiology needs neurobiology—and vice versa. Schizophr Bull 2009; 35: 577–581.
Acknowledgements
The authors acknowledge the support of the National Health and Medical Research Council, the Australian Research Council, and the Queensland Brain Institute. The photograph of C. elegans was kindly provided by Brent Neumann.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Burne, T., Scott, E., van Swinderen, B. et al. Big ideas for small brains: what can psychiatry learn from worms, flies, bees and fish?. Mol Psychiatry 16, 7–16 (2011). https://doi.org/10.1038/mp.2010.35
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2010.35
Keywords
This article is cited by
-
Screening selected medicinal plants for potential anxiolytic activity using an in vivo zebrafish model
Psychopharmacology (2020)
-
Framework for integrating animal welfare into life cycle sustainability assessment
The International Journal of Life Cycle Assessment (2018)
-
The emerging spectrum of allelic variation in schizophrenia: current evidence and strategies for the identification and functional characterization of common and rare variants
Molecular Psychiatry (2013)
-
Transient activation of dopaminergic neurons during development modulates visual responsiveness, locomotion and brain activity in a dopamine ontogeny model of schizophrenia
Translational Psychiatry (2013)
-
Schizophrenia: do all roads lead to dopamine or is this where they start? Evidence from two epidemiologically informed developmental rodent models
Translational Psychiatry (2012)