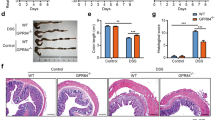
Abstract
The transient receptor potential (TRP) ion channel family is well characterized in sensory neurons; however, little is known about its role in the immune system. Here we show that the cold-sensing TRPM8 has an unexpected role in innate immunity. TRPM8 expression and function in macrophages were demonstrated in vitro using molecular techniques and calcium imaging. In addition, adoptive macrophage transfer and systemic interleukin (IL)-10 overexpression were performed in experimental colitis. TRPM8 activation induced calcium-transients in murine peritoneal macrophages (PM) and bone marrow-derived macrophages of wild-type (WT) but not TRPM8-deficient mice. TRPM8-deficient PM exhibited defective phagocytosis and increased motility compared with those in WT, whereas the opposite effects of TRPM8 activation were induced in WT PM. TRPM8 activation or blockage/genetic deletion induced a anti- or pro-inflammatory macrophage cytokine profile, respectively. WT mice treated with repeated menthol (TRPM8 agonist) enemas were consistently protected from experimental colitis, whereas TRPM8-deficient mice showed increased colitis susceptibility. Adoptive transfer of TRPM8-deficient macrophages aggravated colitis, whereas systemic IL-10 overexpression rescued this phenotype. TRPM8 activation in peptidergic sensory neurons did not affect neuropeptide release from the inflamed colon. TRPM8 in macrophages determines pro- or anti-inflammatory actions by regulating tumor necrosis factor-α and interleukin-10 production. These findings suggest novel TRPM8-based options for immunomodulatory intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Shouval, D.S. et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 40, 706–719 (2014).
Nilius, B. & Owsianik, G. The transient receptor potential family of ion channels. Genome. Biol. 12, 218 (2011).
de Jong, P.R. et al. TRPM8 on mucosal sensory nerves regulates colitogenic responses by innate immune cells via CGRP. Mucosal Immunol. 8, 491–504 (2015).
D'Aldebert, E., Cenac, N. & Rousset, P. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology 140, 275–285 (2011).
Ramachandran, R. et al. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc. Natl. Acad. Sci. USA 110, 7476–7481 (2013).
Engel, M.A. et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology 141, 1346–1358 (2011).
Caceres, A.I. et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc. Natl. Acad. Sci. USA 106, 9099–9104 (2009).
Woolf, C.J. & Ma, Q. Nociceptors—noxious stimulus detectors. Neuron 55, 353–364 (2007).
Tano, J.Y. et al. Impairment of survival signaling and efferocytosis in TRPC3-deficient macrophages. Biochem. Biophys. Res. Commun. 410, 643–647 (2011).
Finney-Hayward, T.K. et al. Expression of transient receptor potential C6 channels in human lung macrophages. Am. J. Respir. Cell Mol. Biol. 43, 296–304 (2010).
Link, T.M. et al. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat. Immunol. 11, 232–239 (2010).
Hamanaka, K. et al. TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 299, 353–362 (2010).
Tsavaler, L. et al. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 61, 3760–3769 (2001).
McKemy, D.D., Neuhausser, W.M. & Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58 (2002).
Peier, A.M. et al. A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715 (2002).
Zhao, C. et al. 1,8-cineol attenuates LPS-induced acute pulmonary inflammation in mice. Inflammation 37, 566–572 (2014).
Juergens, U.R. et al. Inhibitory activity of 1,8-cineol (eucalyptol) on cytokine production in cultured human lymphocytes and monocytes. Pulm. Pharmacol. Ther. 17, 281–287 (2004).
Wu, S.N., Wu, P.Y. & Tsai, M.L. Characterization of TRPM8-like channels activated by the cooling agent icilin in the macrophage cell line RAW 264.7. J. Membr. Biol. 241, 11–20 (2011).
Santos, F.A. et al. 1,8-cineole (eucalyptol), a monoterpene oxide attenuates the colonic damage in rats on acute TNBS-colitis. Food Chem. Toxicol. 42, 579–584 (2004).
Yang, Y. et al. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014, 352371 (2014).
Gabryšová, L. et al. The regulation of IL-10 expression. Curr. Top. Microbiol. Immunol. 380, 157–190 (2014).
Wirtz, S. et al. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2, 541–546 (2007).
Yamamoto, S. et al. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 14, 738–747 (2008).
Knowles, H. et al. Transient Receptor Potential Melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 108, 11578–11583 (2011).
Rozza, A.L. et al. The gastroprotective effect of menthol: involvement of anti-apoptotic, antioxidant and anti-inflammatory activities. PLoS One 9, e86686 (2014).
Mazelin, L. et al. Vagally dependent protective action of calcitonin gene-related peptide on colitis. Peptides 20, 1367–1374 (1999).
Reinshagen, M. et al. Calcitonin gene-related peptide mediates the protective effect of sensory nerves in a model of colonic injury. J. Pharmacol. Exp. Ther. 286, 657–661 (1998).
Abe, K. et al. Conventional dendritic cells regulate the outcome of colonic inflammation independently of T cells. Proc. Natl. Acad. Sci. USA 104, 17022–17027 (2007).
Mueller-Tribbensee, S.M. et al. Differential contribution of TRPA1, TRPV4 and TRPM8 to colonic nociception in mice. PLoS One 10, e0128242 (2015).
Franke, A. et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat. Genet. 40, 1319–1323 (2008).
Doecke, J.D. et al. Genetic susceptibility in IBD: overlap between ulcerative colitis and Crohn's disease. Inflamm. Bowel. Dis. 19, 240–245 (2013).
Mantovani, A. & Marchesi, F. IL-10 and macrophages orchestrate gut homeostasis. Immunity 40, 637–639 (2014).
Zigmond, E. et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 40, 720–733 (2014).
Watanabe, N. et al. Elimination of local macrophages in intestine prevents chronic colitis in interleukin-10-deficient mice. Dig. Dis. Sci. 48, 408–414 (2003).
Tomoyose, M. et al. Role of interleukin-10 in a murine model of dextran sulfate sodium-induced colitis. Scand. J. Gastroenterol. 33, 435–440 (1998).
Saraiva, M. & O'Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 10, 170–181 (2010).
Kelly, E.K., Wang, L. & Ivashkiv, L.B. Calcium-activated pathways and oxidative burst mediate zymosan-induced signaling and IL-10 production in human macrophages. J. Immunol. 184, 5545–5552 (2010).
Vinuela-Fernandez, I. et al. The TRPM8 channel forms a complex with the 5-HT1B receptor and phospholipase D that amplifies its reversal of pain hypersensitivity. Neuropharmacology 79, 136–151 (2013).
Ostacolo, C. et al. Isoxazole derivatives as potent transient receptor potential melastatin type 8 (TRPM8) agonists. Eur. J. Med. Chem. 69, 659–669 (2013).
Rohács, T. et al. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat. Neurosci. 8, 626–634 (2005).
Daniels, R.L., Takashima, Y. & McKemy, D.D. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J. Biol. Chem. 284, 1570–1582 (2009).
Rahimi, R. & Abdollahi, M. Herbal medicines for the management of irritable bowel syndrome: a comprehensive review. World J. Gastroenterol. 18, 589–600 (2012).
Foersch, S., Kiesslich, R. & Waldner, M.J. Molecular imaging of VEGF in gastrointestinal cancer in vivo using confocal laser endomicroscopy. Gut 59, 1046–1055 (2010).
Becker, C., Fantini, M.C. & Neurath, M.F. High resolution colonoscopy in live mice. Nat. Protoc. 1, 2900–2904 (2006).
Van Rooijen, N. & Sanders, A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174, 83–93 (1994).
McHedlidze, T. et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 39, 357–371 (2013).
Liu, X.L., Clark, K.R. & Johnson, P.R. Production of recombinant adeno-associated virus vectors using a packaging cell line and a hybrid recombinant adenovirus. Gene. Ther. 6, 293–299 (1999).
Acknowledgements
We thank I. Izydorczyk, A. Kuhn, J. Schramm, B. Vogler, and S. Haux-Oertel for technical assistance (Institute of Physiology and Pathophysiology). The present work was performed in fulfillment of the requirements for obtaining the degree ‘Dr rer. nat.’ for M. Khalil. M.A.E. received support from the Deutsche Forschungsgemeinschaft (DFG EN 1060/2-1), Marohn-Stiftung, ELAN, IZKF, and Universitätsbund of the Friedrich-Alexander-Universität Erlangen-Nürnberg. P.W.R. was supported by the Federal Ministry of Edu. & Res. (BMBF0315449C).
Author contribution
MK: acquisition of data; analysis and interpretation of data; writing of the manuscript. AB, RL, SF: acquisition of data; analysis and interpretation of data. PWR, SW, CB, MFN: study concept and design; critical revision of the manuscript for important intellectual content. MAE: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; writing of the manuscript; statistical analysis; obtained funding; study supervision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Rights and permissions
About this article
Cite this article
Khalil, M., Babes, A., Lakra, R. et al. Transient receptor potential melastatin 8 ion channel in macrophages modulates colitis through a balance-shift in TNF-alpha and interleukin-10 production. Mucosal Immunol 9, 1500–1513 (2016). https://doi.org/10.1038/mi.2016.16
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2016.16
This article is cited by
-
Uncovering the role of transient receptor potential channels in pterygium: a machine learning approach
Inflammation Research (2023)
-
Transient receptor potential ankyrin 1 promoter methylation and peripheral pain sensitivity in Crohn’s disease
Clinical Epigenetics (2020)