Abstract
Cancerous inhibitor of protein phosphatase 2A (CIP2A) is a predictive biomarker of disease progression in many malignancies, including imatinib-treated chronic myeloid leukemia (CML). Although high CIP2A levels correlate with disease progression in CML, the underlying molecular mechanisms remain elusive. In a screen of diagnostic chronic phase samples from patients with high and low CIP2A protein levels, high CIP2A levels correlate with an antiapoptotic phenotype, characterized by downregulation of proapoptotic BCL-2 family members, including BIM, PUMA and HRK, and upregulation of the antiapoptotic protein BCL-XL. These results suggest that the poor prognosis of patients with high CIP2A levels is due to an antiapoptotic phenotype. Disrupting this antiapoptotic phenotype by inhibition of BCL-XL via RNA interference or A-1331852, a novel, potent and BCL-XL-selective inhibitor, resulted in extensive apoptosis either alone or in combination with imatinib, dasatinib or nilotinib, both in cell lines and in primary CD34+ cells from patients with high levels of CIP2A. These results demonstrate that BCL-XL is the major antiapoptotic survival protein and may be a novel therapeutic target in CML.
Similar content being viewed by others
Introduction
Chronic myeloid leukemia (CML) is a malignant disease of a primitive hematopoietic cell, characterized by a reciprocal translocation between chromosomes 9 and 22 and creates the fusion gene BCR-ABL1, which is a deregulated tyrosine kinase that drives the leukemia.1 CML treatment has been significantly improved by the tyrosine kinase inhibitor (TKI) imatinib, but some patients will eventually fail imatinib treatment and without a change in therapy, a significant proportion will progress towards blast crisis (BC), which is usually rapidly fatal.2, 3 The kinase activity of BCR-ABL is opposed by cellular phosphatases, such as protein phosphatase 2A (PP2A), which is impaired in several malignancies. PP2A plays an important role in regulating cell proliferation, differentiation and apoptosis. In CML, PP2A is inhibited by SET4 and cancerous inhibitor of PP2A (CIP2A).5 CIP2A inhibits PP2A activity and functions by preventing PP2A-driven dephosphorylation and stabilization of c-Myc.6, 7, 8 CIP2A is a strong prospective predictor of subsequent development of BC in imatinib-treated CML patients,5 although the underlying mechanisms remain unclear.
Apoptosis induction is tightly regulated by the BCL-2 family of proteins, which comprise several antiapoptotic members, such as BCL-2, BCL-XL, MCL-1, BCL-w and BFL-1, together with proapoptotic molecules, such as the multidomain effectors BAX and BAK, as well as the BH3-only proteins, including the activators BIM, BID and PUMA, and sensitizers NOXA, HRK, BIK, BMF and BAD.9, 10 The BH3-only members can either be promiscuous or selective with respect to binding their antiapoptotic counterparts. The activators bind all antiapoptotic BCL-2 family members, whereas the sensitizers NOXA and HRK are more selective in binding MCL-1 and BCL-XL, respectively.11 BCR-ABL modulates the expression levels and/or the phosphorylation status of several BCL-2 family members, thus exerting important regulatory effects on apoptosis.12, 13, 14, 15 Furthermore, recent reports suggest important roles for several antiapoptotic BCL-2 family members in CML disease progression.16, 17, 18, 19, 20 Elevated levels of these proteins in several cancers make them promising targets for drug therapy. Small-molecule inhibitors targeting specific members of the BCL-2 family, such as navitoclax/ABT-263 (BCL-2, BCL-XL and BCL-w-specific) and venetoclax/ABT-199 (BCL-2-specific) are in clinical trials for several lymphoid malignancies.21, 22 Recently, selective inhibitors of BCL-XL (A-1331852) and MCL-1 (A-1210477) have been synthesized.23, 24 These inhibitors target the antiapoptotic BCL-2 family members, displacing their sequestered proapoptotic counterparts, thereby resulting in apoptosis.
In this study, we demonstrate both a novel antiapoptotic role for CIP2A in CML pathogenesis and a key role for BCL-XL in survival of CML cell lines and in primary CD34+ cells from patients. These results raise the possibility that inhibition of BCL-XL may be a novel therapeutic option in CML, especially in patients refractory to TKI therapy.
Materials and methods
Reagents and antibodies
Imatinib, nilotinib and dasatinib were from Selleck Chemicals (Houston, TX, USA). ABT-737, ABT-199, A-1331852 and A-1210477 were kindly provided by AbbVie (North Chicago, IL, USA). Antibodies against BIM, PUMA, BMF, BIK, BAD, BCL-XL and BCL-w were from Cell Signaling Technology (Danvers, MA, USA), GAPDH and MCL-1 from Santa Cruz Biotechnology (Santa Cruz, CA, USA), NOXA from Calbiochem (Darmstadt, Germany), BCL-2 from Dako (Ely, UK), BAX and BAK from Millipore (Watford, UK), HRK from Aviva Systems Biology (San Diego, CA, USA) and BID and BFL-1 were from Prof J Borst (The Netherlands Cancer Institute, Amsterdam, The Netherlands). All other reagents, unless mentioned otherwise, were from Sigma-Aldrich (St Louis, MO, USA).
Patient cohort
The study was approved by the Liverpool Central Research Ethics Committee; all 31 patients gave informed consent and were aged 18 or over. All have been seen since original diagnosis of chronic phase CML at our center and have been followed for at least 12 months (median follow-up: 39 months). Patients’ characteristics are presented in Supplementary Table S1.
Sample collection, preparation and cell culture
At diagnosis, mononuclear cells from chronic phase CML patients were separated by density-dependent centrifugation (Lymphoprep Axis-Shield, Oslo, Norway), washed in RPMI 1640 (BioSera, Uckfield, UK) and resuspended in 10% dimethyl sulfoxide/10% fetal calf serum (BioSera)/RPMI at 4 °C and cryopreserved in liquid nitrogen. Wherever possible, samples were enriched for CD34+ cells using the CliniMACS kit (Miltenyi Biotec, Auburn, CA, USA). CD34+ cells were cultured using StemSpan SFEMII media (Stemcell Technologies, Cambridge, UK). K562 and KCL22 cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum and 5 mm l-glutamine.
BH3 profiling and flow cytometry
BH3 profiling was carried out using BH3 peptides from New England Peptide (Gardner, MA, USA) as previously described.25 Loss of mitochondrial membrane potential and apoptosis were quantified by flow cytometry as described.26 Patients with CIP2A levels ⩾7.3 mean fluorescence units by flow cytometry were defined as high CIP2A patients, as every patient that progressed to BC had CIP2A >7.3 mean fluorescence units.27 This cutoff value was derived using receiver operating characteristics (ROC) curve analysis for the prediction of BC based on the diagnostic CIP2A protein level; minimization of the Euclidian distance between the receiver operating characteristics curve and the corner (0, 1) was the criterion used. The optimal cutoff value produced an AUCROC=0.902 (95% CI: 0.832, 0.973).
siRNA knockdowns, immunoprecipitation and western blotting
Cells were reverse-transfected with 10 nm of BAK (s1880 and s1881), BAX (s1888 and s1889), BIM (s195011), PUMA (pool of siRNAs), BMF (pool of siRNAs), BIK (s1989 and s1990), HRK (s194952), BCL-XL (s1920), MCL-1 (s8583), BCL-w (s1924), BFL-1 (pool of siRNAs) from Life Technologies (Paisley, UK), BID (SI02654568), NOXA (SI00129430), BAD (SI00299348), BCL-2 (S100299411) from Qiagen (Manchester, UK) using Interferin (Polyplus Transfection, NY, USA), according to the manufacturer's protocol and processed 48 h after transfection. Immunoprecipitation and western blotting were carried out according to the standard protocols.26
mRNA expression
Quantitative reverse transcription–PCR was performed using cDNA from total leukocytes. Pre-designed TaqMan real-time PCR assays were used for BCL2L11 (Hs00708019_s1), BCL2L1 (Hs00236329_m1), BID (Hs00609632_m1), BBC3 (Hs00248075_m1), HRK (Hs02621354_s1) and BAD (Hs00188930_m1) and GAPDH (Hs99999905_m1) (Life Technologies). PCR was performed using a Stratagene MX3005P PCR machine (Agilent Technologies, Folsom, CA, USA). In evaluating the mRNA expression data, the comparative Ct method was used, with the 2−ΔΔCt formula to achieve results for relative quantification. A pool of cDNA from four normal individuals was used as a calibrator and all samples were normalized to GAPDH.
Statistical analysis
Statistical analysis was conducted using one-way analysis of variance applying the Welch correction and Dunnet’s two-sided multiple comparison test to compare the different treatments to the appropriate control peptide/siRNA (*P⩽0.05, **P⩽0.01, ***P⩽0.001). For continuous variables, the Mann–Whitney U-test was used for comparisons between independent samples. For categorical variables, Fisher’s exact test was used. Progression-free survival functions were estimated by the Kaplan–Meier estimator and the log-rank test was used for comparisons between groups. Statistical analysis was performed using GraphPad Prism (GraphPad Prism Software, Inc., La Jolla, CA, USA).
Results
TKIs prime CML cell lines to undergo apoptosis
Since high levels of CIP2A contributed to imatinib resistance in CML, we wished to understand the role of BCL-2 family members in this resistance mechanism. Using BH3 profiling, a peptide-based technique to determine BCL-2 family dependencies,25 we observed extensive loss of mitochondrial membrane potential (ϕm) in two CML cell lines, K562 and KCL22, following exposure to increasing concentrations of different BH3 peptides (Figure 1a and Supplementary Figure S1). Although all BH3-only activators exhibited extensive mitochondrial depolarization, BH3-only sensitizers demonstrated greater selectivity as demonstrated by a concentration-dependent loss in ϕm following BMF, BAD and HRK, but not NOXA (Figure 1a and Supplementary Figure S1). These results suggested that the survival of these cells depended more on BCL-2, BCL-XL and BCL-w, rather than on MCL-1 and BFL-1, as NOXA was the only sensitizer among the list to specifically target both MCL-1 and BFL-1 (Figure 1a and Supplementary Figure S1).9, 10, 11 In dynamic BH3 profiling studies,28 increasing concentrations of TKIs resulted in a significant loss of ϕm, only when the cells were subsequently exposed to the BIM peptide, suggesting that TKIs primed these cells to apoptosis and a combination therapy with another apoptotic stimuli could facilitate rapid apoptosis in these cells (Figure 1b and Supplementary Figure S2). Since our data implicated specific members of the BCL-2 family in antagonizing apoptosis, we performed RNA interference to silence the expression of different BCL-2 family members to study their effects on TKI-mediated apoptosis (Figure 1c). The concentrations of TKIs used in these studies were determined from their concentration–response curves (Supplementary Figure S3). Downregulation of BCL-XL and to some extent BCL-2 resulted in apoptosis, suggesting that BCL-XL is a critical survival factor in both CML cell lines (Figure 1c). Furthermore, downregulation of BCL-XL, and to a lesser extent, BCL-2 and MCL-1, significantly potentiated TKI-mediated apoptosis in both K562 and KCL22 (Figure 1c), thus confirming an important role for antiapoptotic BCL-2 family members in TKI-mediated apoptosis.
BH3 profiling and RNA interference implicate roles for BCL-2, BCL-XL and MCL-1 in TKI-induced apoptosis. (a) BH3 profiling in K562 cells was carried out using the specified concentrations of different BH3 peptides for 2 h. PUMA-2A was used as the control peptide. (b) Dynamic BH3 profiling in K562 and KCL22 cells, exposed to increasing concentrations of imatinib for 16 h, was carried out using either control peptide (bold continuous lines) or BIM peptide (dotted lines) at 1 μm for 2 h. (c) K562 and KCL22 cells, reverse-transfected with the indicated siRNAs, were exposed for 48 h to dimethyl sulfoxide, imatinib (1 μm), nilotinib (50 nm) or dasatinib (3 nm) and apoptosis assessed by phosphatidylserine (PS) externalization. Statistical analysis was conducted using one-way analysis of variance applying the Welch correction and Dunnet’s two-sided multiple comparison test to compare the different treatments to the appropriate control peptide/ siRNA (*P⩽0.05, **P⩽0.01, ***P⩽0.001). Error bars represent standard error of mean (s.e.m.) from three independent experiments.
TKIs induce apoptosis in a BH3-dependent manner
Exposure to TKIs caused a time-dependent decrease in the expression levels of most anti- and proapoptotic BCL-2 family members, with the notable exception of BAD, which was significantly upregulated (Supplementary Figure S4). To understand the relative contribution of different proapoptotic BCL-2 members in TKI-mediated apoptosis, we silenced the expression of BAX, BAK as well as BH3-only activators and sensitizers in K562 and KCL22 (Figure 2). Although all the proapoptotic effector and activator proteins were critical for TKI-mediated apoptosis, a selective dependence on HRK and BAD, but not NOXA, BMF or BIK was observed in TKI-mediated apoptosis (Figure 2 and Supplementary Figure S4), thus implicating a regulatory role for several BH3-only members in TKI-induced apoptosis.
TKIs induce apoptosis in a BH3-dependent manner. (a, b) K562 and KCL22 cells, reverse-transfected with the indicated siRNAs, were exposed for 48 h to dimethyl sulfoxide, imatinib (1 μm), nilotinib (50 nm) and dasatinib (3 nm) and apoptosis assessed by phosphatidylserine (PS) externalization. Statistical analysis was conducted using one-way analysis of variance applying the Welch correction and Dunnet’s two-sided multiple comparison test to compare the different siRNA transfections to their appropriate control siRNA in each treatment (*P⩽0.05, **P⩽0.01, ***P⩽0.001). Error bars represent s.e.m. from three independent experiments.
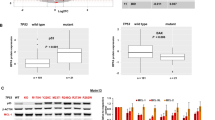
Downregulation of proapoptotic BCL-2 family proteins is associated with disease progression in imatinib-treated patients
To investigate a possible relationship between these BH3-only proteins and clinical outcome, we compared the mRNA expression levels of these proteins with progression-free survival of chronic phase CML patients, treated with imatinib at diagnosis (Figure 3). The median expression level for each gene was calculated and patients were stratified as high or low relative to the median. Low BIM expression was associated with an inferior progression-free survival, whereas BID or BAD expression did not correlate with clinical outcome (Figure 3). Low PUMA and HRK expression were significantly associated with disease progression to BC (P=0.03; Figure 3). In this study, four patients progressed to BC and this disease progression was not associated with the presence of BCR-ABL kinase domain mutations. Low expression of BIM, PUMA and HRK was also associated with poor overall survival but this did not reach significance (data not shown). Moreover, 50% of patients with low PUMA or HRK expression at diagnosis had progressed by 36 months (Figure 3). In addition, low diagnostic levels of BIM and HRK were associated with a slower rate of deep molecular response (MR5) during the first three years of treatment (data not shown).
Expression levels of the proapoptotic BH3-only proteins correlate with progression-free survival in CML patients. Progression-free survival for patients treated with imatinib at initial diagnosis. PCR was performed using total leukocytes collected at initial diagnosis. Patients were stratified into high and low expression groups according to the median mRNA expression for BIM, BID, PUMA, HRK and BAD and the number of cases assessed presented below each graph. The log-rank test was used to determine the significance between high and low expressers.
CIP2A levels correlate with the balance between pro- and antiapoptotic BCL-2 family proteins
Since CML disease progression correlates with high CIP2A levels,5, 29 as well as changes in expression levels of different BCL-2 family members (Figure 3), we speculated whether CIP2A levels could correlate with the expression levels of different BCL-2 family members. To investigate this possibility, we assessed mRNA expression for BIM, BID, PUMA, HRK, BAD and BCL-XL in newly diagnosed chronic phase CML patients. Expression levels of BIM, PUMA and HRK were significantly lower in high compared with low CIP2A patients (Figure 4). A similar trend was observed for BID and BAD expression but this did not reach statistical significance (Figure 4). In contrast, patients with high CIP2A levels expressed high levels of BCL-XL, although this did not reach statistical significance (Figure 4). Taken together these results suggest that CIP2A may exhibit its oncogenic activity by altering the balance of pro- and antiapoptotic proteins resulting in an antiapoptotic phenotype.
High CIP2A expression levels correlate with an antiapoptotic phenotype. mRNA expression for BIM, BID, PUMA, HRK, BAD and BCL-XL in 31 newly diagnosed chronic phase CML patients stratified by their diagnostic CIP2A status. A pool of four normal healthy volunteers was used as a calibrator pool. Statistical analysis was conducted using a Mann–Whitney U-test comparing high and low CIP2A patients (**P⩽0.01). Error bars represent s.e.m.
BCL-XL is a critical survival factor and antagonizes TKI-induced apoptosis in CML cell lines
Since our initial data identified BCL-XL as a critical survival factor in CML cell lines, we used a toolkit of selective BCL-2 family inhibitors, comprising ABT-737 (BCL-2, BCL-XL and BCL-w-specific inhibitor), ABT-199 (BCL-2-selective), A-1331852 (BCL-XL-specific) and A-1210477 (MCL-1-selective) to further evaluate the role of BCL-XL in CML cell survival. In both cell lines, A-1331852 was extremely potent, inducing apoptosis at low nanomolar concentrations, whereas the other inhibitors failed to induce apoptosis even at 100-fold higher concentrations (Figure 5a). A-1331852 was efficient in displacing both BIM and BAD from BCL-XL and releasing them into the cytosol (Figure 5b). Furthermore, A-1331852, but not ABT-199, was efficacious in potentiating TKI-mediated apoptosis for 2G TKIs (Figure 5c). A combination of nilotinib and A-1331852 was more potent than either A-1331852 or nilotinib alone in displacing BIM from BCL-XL (Figure 5d), further suggesting that A-1331852 can be effective in inducing apoptosis in CML cell lines, either as a single agent or in combination with TKIs.
BCL-XL is a critical survival factor and regulates TKI-induced apoptosis in CML cell lines. (a) K562 and KCL22 cells were exposed for 24 h to the specified inhibitors and apoptosis assessed by phosphatidylserine (PS) externalization. (b) Immunoprecipitation of BCL-XL was carried out in K562 cells, exposed to A-1331852 (100 nm) for 0–2 h, and the eluted complexes were immunoblotted for the indicated proteins. The input cell lysates and the immunodepleted supernatant (labeled as Flow-through) were immunoblotted to check the efficiency of the immunoprecipitation. BC represents beads control. (c) K562 cells, exposed for 1 h to 1 nm of ABT-199 or A-1331852, were further exposed in the presence of the pretreated inhibitors to imatinib (1 μm), nilotinib (50 nm) or dasatinib (3 nm) for 24 h and apoptosis assessed. (d) Same as (b) but the immunoprecipitation was carried out with antibodies against BCL-XL in K562 cells exposed to A-1331852 (1 nm) with or without nilotinib (50 nm) for 24 h. Statistical analysis was conducted using one-way analysis of variance applying the Welch correction and Dunnet’s two-sided multiple comparison test to compare the TKI treatments with the combination treatments of A-1331852, represented by the black and gray histograms (*P⩽0.05). Error bars represent s.e.m. from three independent experiments.
A-1331852 exhibits remarkable potency both as a single agent and in combination with TKIs in killing primary CD34+ CML cells
We next investigated the ability of A-1331852 to induce apoptosis in primary CD34+ progenitor cells from high CIP2A patients. In agreement with our data in CML cell lines, A-1331852 displayed remarkable potency in inducing apoptosis in these cells at low nanomolar concentrations as early as 1 h post-treatment (Figure 6a). Prolonged exposure (4 h) resulted in improved potency as A-1331852 induced extensive apoptosis (P=0.002) at concentrations as low as 1 nm in these cells (Figure 6b). Similar results were observed in CD34+ progenitor cells from low CIP2A patients (Supplementary Figure S5). In contrast, mononuclear cells isolated from healthy volunteers generally remained insensitive to the treatment (Figures 6c and d). This is particularly significant as clinically achievable concentrations of imatinib (5 μm), nilotinib (5 μm) or dasatinib (150 nm) did not induce significant apoptosis in primary CD34+ cells after 4 h exposure (data not shown). Even after 24 h exposure, none of these TKIs induced much if any apoptosis above the high spontaneous apoptosis observed in the progenitor cells (Figure 6e). However a subsequent and short exposure to A-1331852 (1 h) following the initial 24 h exposure to TKIs was sufficient to induce enhanced apoptosis in these CD34+ cells (P⩽0.01, Figure 6e). These data support the possibility of targeting BCL-XL, as a novel and effective therapeutic strategy in CML (Figure 7).
Inhibition of BCL-XL promotes rapid apoptosis in primary CML cells. (a, b) Diagnostic chronic phase CD34+ cells from high CIP2A patients were exposed to A-1331852 for 1 h (n=3) and 4 h (n=5) and apoptosis assessed. (c, d) Mononuclear cells (MNCs) from healthy volunteers were exposed to A-1331852 for 1 h (n=8) and 4 h (n=6) and apoptosis assessed. (e) Diagnostic chronic phase CD34+ cells from high CIP2A patients were exposed to imatinib (5 μm), dasatinib (150 nm) and nilotinib (5 μm) for 24 h followed by the addition of A-1331852 (10 nm) to the cells for a further 1 h (n=5). Statistical analysis was conducted using a Mann–Whitney U-test and P-values specified, where significant. Error bars represent s.e.m.
Selective inhibition of BCL-XL overcomes CIP2A-mediated regulation of BCL-2 family members and disease progression in CML. The links that we have established/confirmed in this study are presented as bold lines whereas the dashed lines represent findings from literature. (a) The constitutively active kinase activity of BCR-ABL, antagonized by TKIs, results in phosphorylation of STAT5. (b) p-STAT5 induces the transcription of BCL-XL. (c) BCL-XL sequesters and inhibits BH3-only proteins. (d) This antiapoptotic activity is abolished by A-1331852. (e) Failure to achieve effective apoptosis results in disease progression. (f) High levels of CIP2A correlate with imatinib resistance in CML patients. (g) This can be overcome by second generation (2G) TKIs. (h) CIP2A expression levels correlate with an antiapoptotic phenotype characterized by changes in the balance between the pro- and antiapoptotic BCL-2 family members, thus conferring resistance to TKI therapy in CML. The precise mechanisms by which high CIP2A correlates with the antiapoptotic phenotype is unknown and hence marked with a ‘?’ (i) Selective inhibition of BCL-XL induces rapid apoptosis in CML cells, thus providing a novel and promising therapeutic option.
Discussion
High expression of CIP2A contributes to imatinib resistance in CML and is a strong prospective predictor of subsequent development of BC in imatinib-treated patients.5 However the mechanism(s) by which CIP2A increases the risk of disease progression is poorly understood. In this study, we have identified several proapoptotic BCL-2 family members to be critical in TKI-mediated apoptosis (Figures 1 and 2). These findings also extended to CML patients, as decreased expression of specific proapoptotic BH3-only members PUMA, HRK and possibly BIM correlated with disease progression in CML patients (Figure 3). To our knowledge, this is the first study to link several proapoptotic BCL-2 family members to progression-free survival in imatinib-treated CML patients. We show that high CIP2A expression levels correspond to low expression of specific BH3-only proteins, BIM, PUMA and HRK, and an increase in the expression of BCL-XL (Figure 4), highly characteristic of an antiapoptotic phenotype.
Recently, we have shown that administration of 2G TKIs, such as nilotinib and dasatinib, can overcome high CIP2A and prevent disease progression.5, 27 However, this is not without worrying side effects, as dasatinib has a 25% risk of pleural effusion within ~3 years and nilotinib therapy is associated with hyperglycemia in some patients and a dose-related (8–10%) risk of myocardial infarction, cerebrovascular event or peripheral arterial occlusive event by 6 years.30, 31 This necessitates research into possible alternate therapeutic strategies. In this study, using a BCL-XL-specific inhibitor, A-1331852,23 we demonstrate for the first time, an effective therapeutic option for CML patients with high CIP2A expression levels. A-1331852 displayed remarkable potency, both as a single agent and in combination with TKIs, to induce apoptosis in cell lines and in progenitor CD34+ primary cells (Figures 5 and 6) demonstrating the critical importance of BCL-XL in the survival of CML cells. Although BCL-XL has been associated with disease progression,12, 19, 32, 33, 34 this is the first study that demonstrates a novel antiapoptotic role for CIP2A in CML pathogenesis and how this can be overcome by selectively targeting BCL-XL. This therapeutic option appears particularly promising because the CD34+ progenitor cells were highly sensitive to nanomolar concentrations of A-1331852 but insensitive to even prolonged exposure of the TKIs (Figure 6). This observation is in agreement with previous studies demonstrating a BCL-XL dependence of stem cell survival for human embryonic stem cells as well as non-small-cell lung cancer cells.35, 36 Thus, targeting BCL-XL potentially offers great therapeutic benefits in CML, especially due to the insensitivity of quiescent CD34+ progenitor CML cells to imatinib, which is a major factor in the recurrence of the disease on discontinuation of therapy,17, 37 although it will be necessary to overcome potential toxicities, such as thrombocytopenia, associated with BCL-XL inhibition.22, 38
In summary, we clearly demonstrate that high CIP2A corresponds to an antiapoptotic phenotype, which may contribute to the poor prognosis of CML patients. We have also shown that this antiapoptotic phenotype can be overcome in CML by targeting BCL-XL, thus identifying an effective therapeutic option for CML patients with high expression levels of CIP2A (Figure 7). As high CIP2A levels are also implicated in disease progression in acute myeloid leukemia, breast, bladder, cervical, colon, hepatocellular and lung cancer,39, 40, 41, 42, 43, 44, 45, 46 it will be of interest to ascertain if these tumors also exhibit an antiapoptotic phenotype. Targeting this antiapoptotic phenotype with selective BCL-2 family antagonists may offer novel therapeutic approaches to these malignancies.
References
Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D . The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science 1986; 233: 212–214.
Lucas CM, Wang L, Austin GM, Knight K, Watmough SJ, Shwe KH et al. A population study of imatinib in chronic myeloid leukaemia demonstrates lower efficacy than in clinical trials. Leukemia 2008; 22: 1963–1966.
de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol 2008; 26: 3358–3363.
Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell 2005; 8: 355–368.
Lucas CM, Harris RJ, Giannoudis A, Copland M, Slupsky JR, Clark RE . Cancerous inhibitor of PP2A (CIP2A) at diagnosis of chronic myeloid leukemia is a critical determinant of disease progression. Blood 2011; 117: 6660–6668.
Junttila MR, Puustinen P, Niemelä M, Ahola R, Arnold H, Böttzauw T et al. CIP2A inhibits PP2A in human malignancies. Cell 2007; 130: 51–62.
Khanna A, Böckelman C, Hemmes A, Junttila MR, Wiksten J-P, Lundin M et al. MYC-dependent regulation and prognostic role of CIP2A in gastric cancer. J Natl Cancer Inst 2009; 101: 793–805.
Lucas CM, Harris RJ, Giannoudis A, Clark RE . c-Myc inhibition decreases CIP2A and reduces BCR-ABL1 tyrosine kinase activity in chronic myeloid leukemia. Haematologica 2015; 100: e179–e182.
Kim H, Rafiuddin-Shah M, Tu H-C, Jeffers JR, Zambetti GP, Hsieh JJ-D et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol 2006; 8: 1348–1358.
Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 2006; 9: 351–365.
Ku B, Liang C, Jung JU, Oh B-H . Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res 2011; 21: 627–641.
de Groot RP, Raaijmakers JA, Lammers JW, Koenderman L . STAT5-dependent CyclinD1 and Bcl-xL expression in Bcr-Abl-transformed cells. Mol Cell Biol Res Commun 2000; 3: 299–305.
Horita M, Andreu EJ, Benito A, Arbona C, Sanz C, Benet I et al. Blockade of the Bcr-Abl kinase activity induces apoptosis of chronic myelogenous leukemia cells by suppressing signal transducer and activator of transcription 5-dependent expression of Bcl-xL. J Exp Med 2000; 191: 977–984.
Neshat MS, Raitano AB, Wang HG, Reed JC, Sawyers CL . The survival function of the Bcr-Abl oncogene is mediated by Bad-dependent and -independent pathways: roles for phosphatidylinositol 3-kinase and Raf. Mol Cell Biol 2000; 20: 1179–1186.
Salomoni P, Condorelli F, Sweeney SM, Calabretta B . Versatility of BCR/ABL-expressing leukemic cells in circumventing proapoptotic BAD effects. Blood 2000; 96: 676–684.
Korfi K, Mandal A, Furney SJ, Wiseman D, Somervaille TCP, Marais R . A personalised medicine approach for ponatinib-resistant chronic myeloid leukaemia. Ann Oncol 2015; 26: 1180–1187.
Mak DH, Wang R-Y, Schober WD, Konopleva M, Cortes J, Kantarjian H et al. Activation of apoptosis signaling eliminates CD34+ progenitor cells in blast crisis CML independent of response to tyrosine kinase inhibitors. Leukemia 2012; 26: 788–794.
Ko TK, Chuah CTH, Huang JWJ, Ng K-P, Ong ST . The BCL2 inhibitor ABT-199 significantly enhances imatinib-induced cell death in chronic myeloid leukemia progenitors. Oncotarget 2014; 5: 9033–9038.
Harb JG, Neviani P, Chyla BJ, Ellis JJ, Ferenchak GJ, Oaks JJ et al. Bcl-xL anti-apoptotic network is dispensable for development and maintenance of CML but is required for disease progression where it represents a new therapeutic target. Leukemia 2013; 27: 1996–2005.
Song T, Chai G, Liu Y, Xie M, Chen Q, Yu X et al. Mechanism of synergy of BH3 mimetics and paclitaxel in chronic myeloid leukemia cells: Mcl-1 inhibition. Eur J Pharm Sci 2015; 70: 64–71.
Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008; 68: 3421–3428.
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013; 19: 202–208.
Leverson JD, Phillips DC, Mitten MJ, Boghaert ER, Diaz D, Tahir SK et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med 2015; 7: 279ra40.
Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue J et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis 2015; 6: e1590.
Ryan J, Letai A . BH3 profiling in whole cells by fluorimeter or FACS. Methods 2013; 61: 156–164.
Vogler M, Butterworth M, Majid A, Walewska RJ, Sun X-M, Dyer MJS et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood 2009; 113: 4403–4413.
Lucas CM, Harris RJ, Holcroft AK, Scott LJ, Carmell N, McDonald E et al. Second generation tyrosine kinase inhibitors prevent disease progression in high-risk (high CIP2A) chronic myeloid leukaemia patients. Leukemia 2015; 29: 1514–1523.
Montero J, Sarosiek KA, DeAngelo JD, Maertens O, Ryan J, Ercan D et al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell 2015; 160: 977–989.
Neelakantan P, Gerrard G, Lucas C, Milojkovic D, May P, Wang L et al. Combining BCR-ABL1 transcript levels at 3 and 6 months in chronic myeloid leukemia: implications for early intervention strategies. Blood 2013; 121: 2739–2742.
O'Brien S, Hedgley C, Foroni L, Apperley J, Osborne W, Zwingers T et al. SPIRIT 2: an NCRI randomised study comparing dasatinib with imatinib in patients with newly diagnosed chronic myeloid leukaemia−2 year follow up. Accepted for the European Haematology Association Annual Meeting, : Vienna, June 2015 (oral presentation).
Hughes TP, Larson RA, Kim D-W, Issaragrisil S, le Coutre PD, Lobo C et al. Efficacy and safety of nilotinib (NIL) vs. imatinib (IM) in patients with newly diagnosed chronic myeloid leukaemia in chronic phase (CML-CP): 6-year follow-up of ENESTnd. Accepted for the European Haematology Association Annual Meeting: Vienna, Austria, June 2015 (poster presentation).
Gutiérrez-Castellanos S, Cruz M, Rabelo L, Godínez R, Reyes-Maldonado E, Riebeling-Navarro C . Differences in BCL-X(L) expression and STAT5 phosphorylation in chronic myeloid leukaemia patients. Eur J Haematol 2004; 72: 231–238.
Bewry NN, Nair RR, Emmons MF, Boulware D, Pinilla-Ibarz J, Hazlehurst LA . Stat3 contributes to resistance toward BCR-ABL inhibitors in a bone marrow microenvironment model of drug resistance. Mol Cancer Ther 2008; 7: 3169–3175.
Amarante-Mendes GP, McGahon AJ, Nishioka WK, Afar DE, Witte ON, Green DR . Bcl-2-independent Bcr-Abl-mediated resistance to apoptosis: protection is correlated with up regulation of Bcl-xL. Oncogene 1998; 16: 1383–1390.
Bai H, Chen K, Gao Y-X, Arzigian M, Xie Y-L, Malcosky C et al. Bcl-xL enhances single-cell survival and expansion of human embryonic stem cells without affecting self-renewal. Stem Cell Res 2012; 8: 26–37.
Zeuner A, Francescangeli F, Contavalli P, Zapparelli G, Apuzzo T, Eramo A et al. Elimination of quiescent/slow-proliferating cancer stem cells by Bcl-XL inhibition in non-small cell lung cancer. Cell Death Differ 2014; 21: 1877–1888.
Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ . Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest 2011; 121: 396–409.
Vogler M, Hamali HA, Sun X-M, Bampton ETW, Dinsdale D, Snowden RT et al. BCL2/BCL-X(L) inhibition induces apoptosis, disrupts cellular calcium homeostasis, and prevents platelet activation. Blood 2011; 117: 7145–7154.
Cristóbal I, Garcia-Orti L, Cirauqui C, Alonso MM, Calasanz MJ, Odero MD . PP2A impaired activity is a common event in acute myeloid leukemia and its activation by forskolin has a potent anti-leukemic effect. Leukemia 2011; 25: 606–614.
Come C, Laine A, Chanrion M, Edgren H, Mattila E, Liu X et al. CIP2A is associated with human breast cancer aggressivity. Clin Cancer Res 2009; 15: 5092–5100.
Huang LP, Adelson ME, Mordechai E, Trama JP . CIP2A expression is elevated in cervical cancer. Cancer Biomark 2010; 8: 309–317.
Huang LP, Savoly D, Sidi AA, Adelson ME, Mordechai E, Trama JP . CIP2A protein expression in high-grade, high-stage bladder cancer. Cancer Med 2012; 1: 76–81.
Teng H-W, Yang S-H, Lin J-K, Chen W-S, Lin T-C, Jiang J-K et al. CIP2A is a predictor of poor prognosis in colon cancer. J Gastrointest Surg 2012; 16: 1037–1047.
He H, Wu G, Li W, Cao Y, Liu Y . CIP2A is highly expressed in hepatocellular carcinoma and predicts poor prognosis. Diagn Mol Pathol 2012; 21: 143–149.
Dong Q-Z, Wang Y, Dong X-J, Li Z-X, Tang Z-P, Cui Q-Z et al. CIP2A is overexpressed in non-small cell lung cancer and correlates with poor prognosis. Ann Surg Oncol 2011; 18: 857–865.
De P, Carlson J, Leyland-Jones B, Dey N . Oncogenic nexus of cancerous inhibitor of protein phosphatase 2A (CIP2A): an oncoprotein with many hands. Oncotarget 2014; 5: 4581–4602.
Acknowledgements
We thank AbbVie for inhibitors, Prof. A Letai for expert guidance on BH3 profiling, Prof. J Borst for antibodies and Dr RJ Harris for support and advice. This work was supported by the NorthWest Cancer Research Grants CR994 (GMC) and CR1040 (SV and GMC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
REC has received research funding from Novartis, Bristol Myers Squibb and Pfizer and is a member of the speakers’ bureau for Novartis. All authors report no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lucas, C., Milani, M., Butterworth, M. et al. High CIP2A levels correlate with an antiapoptotic phenotype that can be overcome by targeting BCL-XL in chronic myeloid leukemia. Leukemia 30, 1273–1281 (2016). https://doi.org/10.1038/leu.2016.42
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.42
This article is cited by
-
Exploring the potential of BH3 mimetic therapy in squamous cell carcinoma of the head and neck
Cell Death & Disease (2019)
-
DRP-1 functions independently of mitochondrial structural perturbations to facilitate BH3 mimetic-mediated apoptosis
Cell Death Discovery (2019)
-
BH3-only proteins are dispensable for apoptosis induced by pharmacological inhibition of both MCL-1 and BCL-XL
Cell Death & Differentiation (2019)
-
Apogossypol-mediated reorganisation of the endoplasmic reticulum antagonises mitochondrial fission and apoptosis
Cell Death & Disease (2019)