Abstract
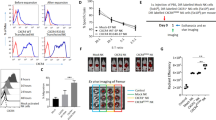
Multiple myeloma (MM) is an incurable hematological malignancy. Chimeric antigen receptor (CAR)-expressing T cells have been demonstrated successfully in the clinic to treat B-lymphoid malignancies. However, the potential utility of antigen-specific CAR-engineered natural-killer (NK) cells to treat MM has not been explored. In this study, we determined whether CS1, a surface protein that is highly expressed on MM cells, can be targeted by CAR NK cells to treat MM. We successfully generated a viral construct of a CS1-specific CAR and expressed it in human NK cells. In vitro, CS1-CAR NK cells displayed enhanced MM cytolysis and interferon-γ (IFN-γ) production, and showed a specific CS1-dependent recognition of MM cells. Ex vivo, CS1-CAR NK cells also showed similarly enhanced activities when responding to primary MM tumor cells. More importantly, in an aggressive orthotopic MM xenograft mouse model, adoptive transfer of NK-92 cells expressing CS1-CAR efficiently suppressed the growth of human IM9 MM cells and also significantly prolonged mouse survival. Thus, CS1 represents a viable target for CAR-expressing immune cells, and autologous or allogeneic transplantation of CS1-specific CAR NK cells may be a promising strategy to treat MM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kyle RA, Rajkumar SV . Multiple myeloma. Blood 2008; 111: 2962–2972.
Siegel R, Naishadham D, Jemal A . Cancer statistics. 2012 CA Cancer J Clin 2012; 62: 10–29.
Palumbo A, Rajkumar SV . Treatment of newly diagnosed myeloma. Leukemia 2009; 23: 449–456.
Podar K, Chauhan D, Anderson KC . Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia 2009; 23: 10–24.
Godfrey J, Benson DM Jr . The role of natural killer cells in immunity against multiple myeloma. Leuk Lymphoma 2012; 53: 1666–1676.
Narni-Mancinelli E, Vivier E, Kerdiles YM . The 'T-cell-ness' of NK cells: unexpected similarities between NK cells and T cells. Int Immunol 2011; 23: 427–431.
Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA . Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010; 18: 843–851.
Porter DL, Levine BL, Kalos M, Bagg A, June CH . Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011; 365: 725–733.
Fauriat C, Mallet F, Olive D, Costello RT . Impaired activating receptor expression pattern in natural killer cells from patients with multiple myeloma. Leukemia 2006; 20: 732–733.
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368: 1509–1518.
Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5: 177ra138.
Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011; 3: 95ra73.
Maus MV, June CH . Zoom Zoom: racing CARs for multiple myeloma. Clin Cancer Res 2013; 19: 1917–1919.
Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res 2008; 14: 2775–2784.
Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 2008; 112: 1329–1337.
Benson DM Jr, Byrd JC . CS1-directed monoclonal antibody therapy for multiple myeloma. J Clin Oncol 2012; 30: 2013–2015.
Tai YT, Soydan E, Song W, Fulciniti M, Kim K, Hong F et al. CS1 promotes multiple myeloma cell adhesion, clonogenic growth, and tumorigenicity via c-maf-mediated interactions with bone marrow stromal cells. Blood 2009; 113: 4309–4318.
van de Donk NW, Kamps S, Mutis T, Lokhorst HM . Monoclonal antibody-based therapy as a new treatment strategy in multiple myeloma. Leukemia 2012; 26: 199–213.
Lonial S, Vij R, Harousseau JL, Facon T, Moreau P, Mazumder A et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol 2012; 30: 1953–1959.
Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS . NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood 2010; 115: 4293–4301.
Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295: 2097–2100.
Yu J, Wei M, Becknell B, Trotta R, Liu S, Boyd Z et al. Pro- and antiinflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity 2006; 24: 575–590.
Yu J, Mao HC, Wei M, Hughes T, Zhang J, Park IK et al. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood 2010; 115: 274–281.
He S, Chu J, Wu LC, Mao H, Peng Y, Alvarez-Breckenridge CA et al. MicroRNAs activate natural killer cells through Toll-like receptor signaling. Blood 2013; 121: 4663–4671.
Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol 2004; 5: 1260–1265.
Tu SP, Quante M, Bhagat G, Takaishi S, Cui G, Yang XD et al. IFN-gamma inhibits gastric carcinogenesis by inducing epithelial cell autophagy and T-cell apoptosis. Cancer Res 2011; 71: 4247–4259.
Ma J, Chen T, Mandelin J, Ceponis A, Miller NE, Hukkanen M et al. Regulation of macrophage activation. Cell Mol Life Sci 2003; 60: 2334–2346.
Borrego F, Pena J, Solana R . Regulation of CD69 expression on human natural killer cells: differential involvement of protein kinase C and protein tyrosine kinases. Eur J Immunol 1993; 23: 1039–1043.
Phillips JH, Le AM, Lanier LL . Natural killer cells activated in a human mixed lymphocyte response culture identified by expression of Leu-11 and class II histocompatibility antigens. J Exp Med 1984; 159: 993–1008.
Spits H, Lanier LL . Natural killer or dendritic: what's in a name? Immunity 2007; 26: 11–16.
Koehler H, Kofler D, Hombach A, Abken H . CD28 costimulation overcomes transforming growth factor-beta-mediated repression of proliferation of redirected human CD4+ and CD8+ T cells in an antitumor cell attack. Cancer Res 2007; 67: 2265–2273.
Krzewski K, Coligan JE . Human NK cell lytic granules and regulation of their exocytosis. Front Immunol 2012; 3: 335.
Francisco JA, Donaldson KL, Chace D, Siegall CB, Wahl AF . Agonistic properties and in vivo antitumor activity of the anti-CD40 antibody SGN-14. Cancer Res 2000; 60: 3225–3231.
Kochenderfer JN, Rosenberg SA . Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol 2013; 10: 267–276.
Mateo G, Montalban MA, Vidriales MB, Lahuerta JJ, Mateos MV, Gutierrez N et al. Prognostic value of immunophenotyping in multiple myeloma: a study by the PETHEMA/GEM cooperative study groups on patients uniformly treated with high-dose therapy. J Clin Oncol 2008; 26: 2737–2744.
Mihara K, Bhattacharyya J, Kitanaka A, Yanagihara K, Kubo T, Takei Y et al. T-cell immunotherapy with a chimeric receptor against CD38 is effective in eliminating myeloma cells. Leukemia 2012; 26: 365–367.
Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res 2013; 19: 2048–2060.
Laabi Y, Gras MP, Carbonnel F, Brouet JC, Berger R, Larsen CJ et al. A new gene, BCM, on chromosome 16 is fused to the interleukin 2 gene by a t(4;16)(q26;p13) translocation in a malignant T cell lymphoma. EMBO J 1992; 11: 3897–3904.
Laabi Y, Gras MP, Brouet JC, Berger R, Larsen CJ, Tsapis A . The BCMA gene, preferentially expressed during B lymphoid maturation, is bidirectionally transcribed. Nucleic Acids Res 1994; 22: 1147–1154.
O'Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med 2004; 199: 91–98.
Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev 2008; 88: 841–886.
van de Donk NW, Lokhorst HM . New developments in the management and treatment of newly diagnosed and relapsed/refractory multiple myeloma patients. Expert Opin Pharmacother 2013; 14: 1569–1573.
Bae J, Song W, Smith R, Daley J, Tai YT, Anderson KC et al. A novel immunogenic CS1-specific peptide inducing antigen-specific cytotoxic T lymphocytes targeting multiple myeloma. Br J Haematol 2012; 157: 687–701.
Jena B, Dotti G, Cooper LJ . Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood 2010; 116: 1035–1044.
Kruschinski A, Moosmann A, Poschke I, Norell H, Chmielewski M, Seliger B et al. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proc Natl Acad Sci USA 2008; 105: 17481–17486.
Imai C, Iwamoto S, Campana D . Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 2005; 106: 376–383.
Kroger N, Shaw B, Iacobelli S, Zabelina T, Peggs K, Shimoni A et al. Comparison between antithymocyte globulin and alemtuzumab and the possible impact of KIR-ligand mismatch after dose-reduced conditioning and unrelated stem cell transplantation in patients with multiple myeloma. Br J Haematol 2005; 129: 631–643.
Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B . Graft-versus-myeloma effect: proof of principle. Blood 1996; 87: 1196–1198.
Alici E, Konstantinidis KV, Sutlu T, Aints A, Gahrton G, Ljunggren HG et al. Anti-myeloma activity of endogenous and adoptively transferred activated natural killer cells in experimental multiple myeloma model. Exp Hematol 2007; 35: 1839–1846.
Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy 2012; 14: 1131–1143.
Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 2009; 69: 4010–4017.
Tonn T BS, Esser R, Schwabe D, Seifried E . Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res 2001; 10: 535–544.
Swift BE, Williams BA, Kosaka Y, Wang XH, Medin JA, Viswanathan S et al. Natural killer cell lines preferentially kill clonogenic multiple myeloma cells and decrease myeloma engraftment in a bioluminescent xenograft mouse model. Haematologica 2012; 97: 1020–1028.
Cheng M, Chen Y, Xiao W, Sun R, Tian Z . NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol 2013; 10: 230–252.
Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy 2008; 10: 625–632.
Maki G, Hayes GM, Naji A, Tyler T, Carosella ED, Rouas-Freiss N et al. NK resistance of tumor cells from multiple myeloma and chronic lymphocytic leukemia patients: implication of HLA-G. Leukemia 2008; 22: 998–1006.
Bellucci R, Nguyen HN, Martin A, Heinrichs S, Schinzel AC, Hahn WC et al. Tyrosine kinase pathways modulate tumor susceptibility to natural killer cells. J Clin Invest 2012; 122: 2369–2383.
Benson DM Jr, Hofmeister CC, Padmanabhan S, Suvannasankha A, Jagannath S, Abonour R et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood 2012; 120: 4324–4333.
Acknowledgements
The project was supported in part by Multiple Myeloma Opportunities for Research and Education (MMORE), and grants from the National Institutes of Health (CA155521 and 1R41OD018403 to JY and CA095426 and CA068458 to MAC), a 2012 scientific research grant from the National Blood Foundation (JY), an institutional research grant (IRG-67-003-47) from the American Cancer Society (JY), and The Ohio State University Comprehensive Cancer Center Pelotonia grant (JY).
AUTHOR CONTRIBUTIONS
JC designed research, performed experiments, and wrote the paper; YD, SH, YP, HM and LY performed experiments; DMB, KG, XH, SMD, XZ and MAC designed the research; TH reviewed and edited the manuscript; JZ analyzed the data; CCH devised the concept, designed the research and reviewed the manuscript; JY devised the concept, designed the research, supervised the study, and wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Chu, J., Deng, Y., Benson, D. et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 28, 917–927 (2014). https://doi.org/10.1038/leu.2013.279
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.279
Keywords
This article is cited by
-
Targeted immunotherapy: harnessing the immune system to battle multiple myeloma
Cell Death Discovery (2024)
-
Development of NK cell-based cancer immunotherapies through receptor engineering
Cellular & Molecular Immunology (2024)
-
Bispecific CS1-BCMA CAR-T cells are clinically active in relapsed or refractory multiple myeloma
Leukemia (2024)
-
Chimeric antigen receptor engineered natural killer cells for cancer therapy
Experimental Hematology & Oncology (2023)
-
New cell sources for CAR-based immunotherapy
Biomarker Research (2023)