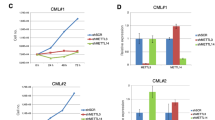
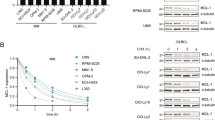
Abstract
Y-box binding protein 1 (YB-1) functions as a translational regulator and has been suggested to elevate MYC mRNA translation via an internal ribosome entry segment (IRES) point mutation in multiple myeloma (MM). We show that YB-1-mediated translation of MYC mRNA occurs independently of the reported IRES mutation, as 87 MM patients (n=88) and all tested human MM cell lines (HMCLs) were negative for the mutation. We show for the first time that positive MYC staining predicts YB-1 co-expression in malignant plasma cells and YB-1/MYC co-expression increases from 30% in medullary to 70% in extramedullary MM. YB-1 knockdown in HMCLs reduced both MYC protein levels and MYC mRNA in the polysomal fraction, providing a mechanism by which YB-1 controls MYC translation. MYC transcription of YB-1 is demonstrated in HMCLs as MYC knockdown resulted in reduced YB-1 protein and mRNA levels. Furthermore, MYC activation in non-malignant mouse embryonic fibroblasts (MEFs) increased YB-1 mRNA, clearly indicating that MYC drives YB-1 transcription. Importantly, perturbation of the MYC/YB-1 oncogenic circuit leads to apoptosis in HMCLs. Here, we demonstrate that these two proteins co-regulate each other via combined transcriptional/translational activity establishing their pivotal role in MM cell survival. We therefore suggest that targeting the YB-1/mRNA interaction provides a new strategy for MM drug development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rajkumar SV . Treatment of multiple myeloma. Nat Rev Clin Oncol 2011; 8: 479–491.
MacDonald GH, Itoh-Lindstrom Y, Ting JP . The transcriptional regulatory protein, YB-1, promotes single-stranded regions in the DRA promoter. J Biol Chem 1995; 270: 3527–3533.
Homer C, Knight DA, Hananeia L, Sheard P, Risk J, Lasham A et al. Y-box factor YB1 controls p53 apoptotic function. Oncogene 2005; 24: 8314–8325.
Rangan VS, Oskouian B, Smith S . Identification of an inverted CCAAT box motif in the fatty-acid synthase gene as an essential element for modification of transcriptional regulation by cAMP. J Biol Chem 1996; 271: 2307–2312.
Bargou RC, Jurchott K, Wagener C, Bergmann S, Metzner S, Bommert K et al. Nuclear localization and increased levels of transcription factor YB-1 in primary human breast cancers are associated with intrinsic MDR1 gene expression. Nat Med 1997; 3: 447–450.
Shibahara K, Sugio K, Osaki T, Uchiumi T, Maehara Y, Kohno K et al. Nuclear expression of the Y-box binding protein, YB-1, as a novel marker of disease progression in non-small cell lung cancer. Clin Cancer Res 2001; 7: 3151–3155.
To K, Fotovati A, Reipas KM, Law JH, Hu K, Wang J et al. Y-box binding protein-1 induces the expression of CD44 and CD49f leading to enhanced self-renewal, mammosphere growth, and drug resistance. Cancer Res 2010; 70: 2840–2851.
Bergmann S, Royer-Pokora B, Fietze E, Jurchott K, Hildebrandt B, Trost D et al. YB-1 provokes breast cancer through the induction of chromosomal instability that emerges from mitotic failure and centrosome amplification. Cancer Res 2005; 65: 4078–4087.
Chatterjee M, Rancso C, Stuhmer T, Eckstein N, Andrulis M, Gerecke C et al. The Y-box binding protein YB-1 is associated with progressive disease and mediates survival and drug resistance in multiple myeloma. Blood 2008; 111: 3714–3722.
Park SS, Shaffer AL, Kim JS, duBois W, Potter M, Staudt LM et al. Insertion of Myc into Igh accelerates peritoneal plasmacytomas in mice. Cancer Res 2005; 65: 7644–7652.
Suematsu S, Matsusaka T, Matsuda T, Ohno S, Miyazaki J, Yamamura K et al. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc Natl Acad Sci USA 1992; 89: 232–235.
Potter M . Neoplastic development in plasma cells. Immunol Rev 2003; 194: 177–195.
Kovalchuk AL, Kim JS, Park SS, Coleman AE, Ward JM, Morse HC et al. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci USA 2002; 99: 1509–1514.
Chng WJ, Huang GF, Chung TH, Ng SB, Gonzalez-Paz N, Troska-Price T et al. Clinical and biological implications of MYC activation: a common difference between MGUS and newly diagnosed multiple myeloma. Leukemia 2011; 25: 1026–1035.
Skopelitou A, Hadjiyannakis M, Tsenga A, Theocharis S, Alexopoulou V, Kittas C et al. Expression of C-myc p62 oncoprotein in multiple myeloma: an immunohistochemical study of 180 cases. Anticancer Res 1993; 13: 1091–1095.
Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011; 146: 904–917.
Cobbold LC, Wilson LA, Sawicka K, King HA, Kondrashov AV, Spriggs KA et al. Upregulated c-myc expression in multiple myeloma by internal ribosome entry results from increased interactions with and expression of PTB-1 and YB-1. Oncogene 2010; 29: 2884–2891.
Chappell SA, LeQuesne JP, Paulin FE, deSchoolmeester ML, Stoneley M, Soutar RL et al. A mutation in the c-myc-IRES leads to enhanced internal ribosome entry in multiple myeloma: a novel mechanism of oncogene de-regulation. Oncogene 2000; 19: 4437–4440.
Landsman D . RNP-1, an RNA-binding motif is conserved in the DNA-binding cold shock domain. Nucl Acids Res 1992; 20: 2861–2864.
Kloks CP, Spronk CA, Lasonder E, Hoffmann A, Vuister GW, Grzesiek S et al. The solution structure and DNA-binding properties of the cold-shock domain of the human Y-box protein YB-1. J Mol Biol 2002; 316: 317–326.
Evdokimova V, Ruzanov P, Imataka H, Raught B, Svitkin Y, Ovchinnikov LP et al. The major mRNA-associated protein YB-1 is a potent 5' cap-dependent mRNA stabilizer. EMBO J 2001; 20: 5491–5502.
Bader AG, Vogt PK . Phosphorylation by Akt disables the anti-oncogenic activity of YB-1. Oncogene 2008; 27: 1179–1182.
Ashizuka M, Fukuda T, Nakamura T, Shirasuna K, Iwai K, Izumi H et al. Novel translational control through an iron-responsive element by interaction of multifunctional protein YB-1 and IRP2. Mol Cell Biol 2002; 22: 6375–6383.
Murphy DJ, Junttila MR, Pouyet L, Karnezis A, Shchors K, Bui DA et al. Distinct thresholds govern Myc's biological output in vivo. Cancer Cell 2008; 14: 447–457.
Kunder S, Calzada-Wack J, Holzlwimmer G, Muller J, Kloss C, Howat W et al. A comprehensive antibody panel for immunohistochemical analysis of formalin-fixed, paraffin-embedded hematopoietic neoplasms of mice: analysis of mouse specific and human antibodies cross-reactive with murine tissue. Toxicol Pathol 2007; 35: 366–375.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550.
Paulin FE, West MJ, Sullivan NF, Whitney RL, Lyne L, Willis AE . Aberrant translational control of the c-myc gene in multiple myeloma. Oncogene 1996; 13: 505–513.
Ponten F, Jirstrom K, Uhlen M . The Human Protein Atlas--a tool for pathology. J Pathol 2008; 216: 387–393.
Dong J, Akcakanat A, Stivers DN, Zhang J, Kim D, Meric-Bernstam F . RNA-binding specificity of Y-box protein 1. RNA Biol 2009; 6: 59–64.
Skabkina OV, Lyabin DN, Skabkin MA, Ovchinnikov LP . YB-1 autoregulates translation of its own mRNA at or prior to the step of 40S ribosomal subunit joining. Mol Cell Biol 2005; 25: 3317–3323.
Tanaka T, Ohashi S, Funakoshi T, Kobayashi S . YB-1 binds to GluR2 mRNA and CaM1 mRNA in the brain and regulates their translational levels in an activity-dependent manner. Cell Mol Neurobiol 2010; 30: 1089–1100.
Evdokimova VM, Kovrigina EA, Nashchekin DV, Davydova EK, Hershey JW, Ovchinnikov LP . The major core protein of messenger ribonucleoprotein particles (p50) promotes initiation of protein biosynthesis in vitro. J Biol Chem 1998; 273: 3574–3581.
Schuhmacher M, Staege MS, Pajic A, Polack A, Weidle UH, Bornkamm GW et al. Control of cell growth by c-Myc in the absence of cell division. Curr Biol 1999; 9: 1255–1258.
Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P et al. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucl Acids Res 1999; 27: 4324–4327.
Cobbold LC, Spriggs KA, Haines SJ, Dobbyn HC, Hayes C, de Moor CH et al. Identification of internal ribosome entry segment (IRES)-trans-acting factors for the Myc family of IRESs. Mol Cell Biol 2008; 28: 40–49.
Shiota M, Izumi H, Onitsuka T, Miyamoto N, Kashiwagi E, Kidani A et al. Twist promotes tumor cell growth through YB-1 expression. Cancer Res 2008; 68: 98–105.
Acknowledgements
We would like to thank C Linden, Institute for Immunobiology, for cell sorting, M Göbel, IZKF Würzburg, for the Bioanalyzer runs; G Bornkamm for the P493-6 cell line; M Nikiforov and M Chatterjee for plasmids encoding shRNA against MYC and YB-1, respectively; and N Königl for technical assistance. This work was supported by the Deutsche Forschungsgemeinshaft (DFG, CRU 216 and SFB-TR 17), by the Interdisciplinary Centre for Clinical Research (IZKF) of Würzburg University and by the Multiple Myeloma Research Foundation (MMRF, USA).
AUTHOR CONTRIBUTIONS
KSB and KB designed, performed and analysed experiments, and contributed to writing the manuscript; RB reviewed the manuscript; ME designed, performed and analysed experiments in HMCLs, and contributed to the writing of the manuscript; EL, SW, ME and AR performed and analysed the IP experiments, EL contributed to the writing of the manuscript. MK and ME performed and analysed density gradient centrifugation. DM and ME performed and analysed the data on MEFs and carefully reviewed the manuscript; CL was responsible for the studies on the point mutation in the MYC IRES, and reviewed the manuscript; SJ provided transgenic mouse tissue samples and reviewed the manuscript. AM and AR stained and evaluated MM material, RM provided and evaluated extramedullary plasmacytomas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Bommert, K., Effenberger, M., Leich, E. et al. The feed-forward loop between YB-1 and MYC is essential for multiple myeloma cell survival. Leukemia 27, 441–450 (2013). https://doi.org/10.1038/leu.2012.185
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.185
Keywords
This article is cited by
-
Stabilization of KPNB1 by deubiquitinase USP7 promotes glioblastoma progression through the YBX1-NLGN3 axis
Journal of Experimental & Clinical Cancer Research (2024)
-
Non-canonical role for the ataxia-telangiectasia-Rad3 pathway in STAT3 activation in human multiple myeloma cells
Cellular Oncology (2023)
-
YB-1 activating cascades as potential targets in KRAS-mutated tumors
Strahlentherapie und Onkologie (2023)
-
LncRNA HOXC-AS3 promotes non-small-cell lung cancer growth and metastasis through upregulation of YBX1
Cell Death & Disease (2022)
-
Targeting USP47 overcomes tyrosine kinase inhibitor resistance and eradicates leukemia stem/progenitor cells in chronic myelogenous leukemia
Nature Communications (2021)