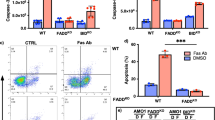
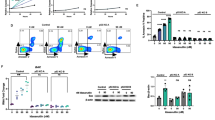
Abstract
t(17;19)-acute lymphoblastic leukemia (ALL) shows extremely poor prognosis. E2A-HLF derived from t(17;19) blocks apoptosis induced by the intrinsic mitochondrial pathway and has a central role in leukemogenesis and chemoresistance. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is expressed on cytotoxic T cells and natural killer cells and binds with death receptors (DR4/DR5), inducing apoptosis by dual activation of intrinsic and extrinsic pathways, and TRAIL mediates the graft-versus-leukemia (GVL) effect after allogeneic stem cell transplantation (allo-SCT). We found that cell lines and patients' samples of t(17;19)-ALL expressed death receptors for TRAIL, and recombinant soluble TRAIL immediately induced apoptosis into t(17;19)-ALL cell lines. E2A-HLF induced gene expression of DR4/DR5, which was dependent on the DNA-binding and transactivation activities of E2A-HLF through the 5′ upstream region of the start site at least in the DR4 gene. Introduction of E2A-HLF into non-t(17;19)-ALL cell line upregulated DR4 and DR5 expression, and sensitized to proapoptotic activity of recombinant soluble TRAIL. Finally, a newly diagnosed t(17;19)-ALL patient underwent allo-SCT immediately after induction of first complete remission, and the patient has survived without relapse for over 3–1/2 years after allo-SCT. These findings suggest that E2A-HLF sensitizes t(17;19)-ALL to the GVL effect by upregulating death receptors for TRAIL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kolb HJ, Schmid C, Barrett AJ, Schendel DJ . Graft-versus-leukemia reactions in allogeneic chimeras. Blood 2004; 103: 767–776.
Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S et al. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 1994; 265: 528–530.
Lowin B, Hahne M, Mattmann C, Tschopp J . Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature 1994; 370: 650–652.
Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J Exp Med 2001; 193: 661–670.
Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med 2001; 7: 94–100.
Schmaltz C, Alpdogan O, Kappel BJ, Muriglan SJ, Rotolo JA, Ongchin J et al. T cells require TRAIL for optimal graft-versus-tumor activity. Nat Med 2002; 8: 1433–1437.
Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J et al. The receptor for the cytotoxic ligand TRAIL. Science 1997; 276: 111–113.
Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM . An antagonist decoy receptor and death domain-containing receptor for TRAIL. Science 1997; 277: 815–818.
Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 1997; 277: 818–821.
Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG . The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 1997; 7: 813–820.
Sprick MR, Weigand MA, Rieser E, Rauch CT, Juo P, Blenis J et al. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 2000; 12: 599–609.
Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A . Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity 2000; 12: 611–620.
Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 1999; 5: 157–163.
Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 1999; 104: 155–162.
Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM . The biology of chronic myeloid leukemia. N Engl J Med 1999; 341: 164–172.
Arico M, Valsecchi MG, Camitta B, Schrappe M, Chessells J, Baruchel A et al. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med 2000; 342: 998–1006.
Uno K, Inukai T, Kayagaki N, Goi K, Sato H, Nemoto A et al. TNF-related apoptosis-inducing ligand (TRAIL) frequently induces apoptosis in Philadelphia chromosome-positive leukemia cells. Blood 2003; 101: 3658–3667.
Pui CH, Gaynon PS, Boyett JM, Chessells JM, Baruchel A, Kamps W et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet 2002; 359: 1909–1915.
Inukai T, Zhang X, Goto M, Hirose K, Uno K, Akahane K et al. Resistance of infant leukemia with MLL rearrangement to tumor necrosis factor-related apoptosis-inducing ligand: a possible mechanism for poor sensitivity to antitumor immunity. Leukemia 2006; 20: 2119–2129.
Look AT . Oncogenic transcription factors in the human acute leukemias. Science 1997; 278: 1059–1064.
Hirose K, Inukai T, Kikuchi J, Furukawa Y, Ikawa T, Kawamoto H et al. Aberrant induction of LMO2 by the E2A-HLF chimeric transcription factor and its implication in leukemogenesis of B-precursor ALL with t(17;19). Blood 2010; 116: 962–970.
Akahane K, Inukai T, Inaba T, Kurosawa H, Look AT, Kiyokawa N et al. Specific induction of CD33 expression by E2A-HLF: the first evidence for aberrant myeloid antigen expression in ALL by a fusion transcription factor. Leukemia 2010; 24: 865–869.
Inukai T, Hirose K, Inaba T, Kurosawa H, Hama A, Inada H et al. Hypercalcemia in childhood acute lymphoblastic leukemia: frequent implication of parathyroid hormone-related peptide and E2A-HLF from translocation 17;19. Leukemia 2007; 21: 288–296.
Kurosawa H, Goi K, Inukai T, Inaba T, Chang KS, Shinjyo T et al. Two candidate downstream target genes for E2A-HLF. Blood 1999; 93: 321–332.
Matsunaga T, Inaba T, Matsui H, Okuya M, Miyajima A, Inukai T et al. Regulation of annexin II by cytokine-initiated signaling pathways and E2A-HLF oncoprotein. Blood 2004; 103: 3185–3191.
Hunger SP . Chromosomal translocations involving the E2A gene in acute lymphoblastic leukemia: clinical features and molecular pathogenesis. Blood 1996; 87: 1211–1224.
Inaba T, Roberts WM, Shapiro LH, Jolly KM, Raimondi SC, Smith SD et al. Fusion of the leucine zipper gene HLF to the E2A gene in human acute B-lineage leukemia. Science 1992; 257: 531–534.
Hunger SP, Ohyashiki K, Toyama K, Cleary ML . Hlf a novel hepatic bZIP protein, shows altered DNA-binding properties following fusion to E2A in t(17;19) acute lymphoblastic leukemia. Genes Dev 1992; 6: 1608–1620.
Inaba T, Shapiro LH, Funabiki T, Sinclair AE, Jones BG, Ashmun RA et al. DNA-binding specificity and trans-activating potential of the leukemia-associated E2A-hepatic leukemia factor fusion protein. Mol Cell Biol 1994; 14: 3403–3413.
Hunger SP, Brown R, Cleary ML . DNA-binding and transcriptional regulatory properties of hepatic leukemia factor (HLF) and the t(17;19) acute lymphoblastic leukemia chimera E2A-HLF. Mol Cell Biol 1994; 14: 5986–5996.
Inukai T, Inaba T, Yoshihara T, Look AT . Cell transformation mediated by homodimeric E2A-HLF transcription factors. Mol Cell Biol 1997; 17: 1417–1424.
Inaba T, Inukai T, Yoshihara T, Seyschab H, Ashmun RA, Canman CE et al. Reversal of apoptosis by the leukaemia-associated E2A-HLF chimaeric transcription factor. Nature 1996; 382: 541–544.
Inukai T, Inaba T, Ikushima S, Look AT . The AD1 and AD2 transactivation domains of E2A are essential for the antiapoptotic activity of the chimeric oncoprotein E2A-HLF. Mol Cell Biol 1998; 18: 6035–6043.
Inukai T, Inaba T, Dang J, Kuribara R, Ozawa K, Miyajima A et al. TEF, an antiapoptotic bZIP transcription factor related to the oncogenic E2A-HLF chimera, inhibits cell growth by down-regulating expression of the common beta chain of cytokine receptors. Blood 2005; 105: 4437–4444.
Altura RA, Inukai T, Ashmun RA, Zambetti GP, Roussel MF, Look AT . The chimeric E2A-HLF transcription factor abrogates p53-induced apoptosis in myeloid leukemia cells. Blood 1998; 92: 1397–1405.
Devaraj PE, Foroni L, Sekhar M, Butler T, Wright F, Mehta A et al. E2A/HLF fusion cDNAs and the use of RT-PCR for the detection of minimal residual disease in t(17;19)(q22;p13) acute lymphoblastic leukemia. Leukemia 1994; 8: 1131–1138.
Akahane K, Inukai T, Zhang X, Hirose K, Kuroda I, Honna H et al. Resistance of T-cell acute lymphoblastic leukemia to tumor necrosis factor--related apoptosis-inducing ligand-mediated apoptosis. Exp Hematol 2010; 38: 885–895.
Yoshida T, Maeda A, Tani N, Sakai T . Promoter structure and transcription initiation sites of the human death receptor 5/TRAIL-R2 gene. FEBS Lett 2001; 507: 381–385.
Wu GS, Burns TF, McDonald ER, Jiang W, Meng R, Krantz ID et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet 1997; 17: 141–143.
Liu X, Yue P, Khuri FR, Sun SY . p53 upregulates death receptor 4 expression through an intronic p53 binding site. Cancer Res 2004; 64: 5078–5083.
Shetty S, Graham BA, Brown JG, Hu X, Vegh-Yarema N, Harding G et al. Transcription factor NF-kappaB differentially regulates death receptor 5 expression involving histone deacetylase 1. Mol Cell Biol 2005; 25: 5404–5416.
Mendoza FJ, Ishdorj G, Hu X, Gibson SB . Death receptor-4(DR4) expression is regulated by transcription factor NF-kappaB in response to etoposide treatment. Apoptosis 2008; 13: 756–770.
Guan B, Yue P, Lotan R, Sun SY . Evidence that the human death receptor 4 is regulated by activator protein 1. Oncogene 2002; 21: 3121–3129.
Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Kayagaki N, Yagita H et al. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med 2002; 195: 161–169.
Baader E, Toloczko A, Fuchs U, Schmid I, Beltinger C, Ehrhardt H et al. Tumor necrosis factor-related apoptosis-inducing ligand-mediated proliferation of tumor cells with receptor-proximal apoptosis defects. Cancer Res 2005; 65: 7888–7895.
Belyanskaya LL, Ziogas A, Hopkins-Donaldson S, Kurtz S, Simon HU, Stahel R et al. TRAIL-induced survival and proliferation of SCLC cells is mediated by ERK and dependent on TRAIL-R2/DR5 expression in the absence of caspase-8. Lung Cancer 2008; 60: 355–365.
Ruiz-Ruiz C, Muñoz-Pinedo C, López-Rivas A . Interferon-gamma treatment elevates caspase-8 expression and sensitizes human breast tumor cells to a death receptor-induced mitochondria-operated apoptotic program. Cancer Res 2000; 60: 5673–5680.
Kanetaka Y, Hayashida M, Hoshika A, Yanase N, Mizuguchi J . Interferon-alpha induces transient upregulation of c-FLIP through NF-kappaB activation. Exp Cell Res 2008; 314: 246–254.
Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol 2007; 25: 1390–1395.
Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res 2007; 13: 6187–6194.
Acknowledgements
We thank H Goto (Department of Pediatrics, Yokohama City University, Japan) for YCUB-2 cell line, F Rauscher III (Wister Institute, PA) for the pMT-CB6+ expression vector and T Sakai (Department of Molecular-Targeting Cancer Prevention, Kyoto Prefectural University of Medicine, Japan) for the vectors for DR5 reporter assay. This work was supported in part by research grants from the Ministry of Education, Science and Culture in Japan.
Author contributions
XZ performed experiments and analyzed the data; TI designed the project, analyzed the data and wrote the manuscript; KH, KA, IK, HH, KK, KG, performed experiments; KN, MK, ME provided critical tools for analysis; HY, HK, HH provided critical tools for analysis and wrote the manuscript; ATL designed and supervised the project; HH provided critical tools for analysis; TI designed the project and wrote the manuscript; SN supervised the project; KS supervised the project and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, X., Inukai, T., Hirose, K. et al. Oncogenic fusion E2A-HLF sensitizes t(17;19)-positive acute lymphoblastic leukemia to TRAIL-mediated apoptosis by upregulating the expression of death receptors. Leukemia 26, 2483–2493 (2012). https://doi.org/10.1038/leu.2012.139
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.139
Keywords
This article is cited by
-
Clonal evolution in adult TCF3::HLF-positive acute lymphoblastic leukemia undergoing stem cell transplantation
Annals of Hematology (2022)
-
While at Rome miRNA and TRAIL Do Whatever BCR-ABL Commands to Do
Archivum Immunologiae et Therapiae Experimentalis (2013)