Abstract
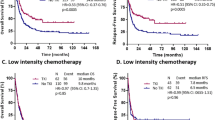
High FLT3-ITD/wildtype (wt) load in FLT3-ITD-mutated AML has been associated with adverse impact on outcome in several studies. To clarify whether FLT3-ITD load as expressed as FLT3-ITD/wt ratio is also relevant in patients with NPM1 mutated AML, we assessed the FLT3-ITD mutation status and FLT3-ITD/wt ratio by fragment analysis in 638 NPM1mut AML (339 females; 299 males; 17.8–88.0 years), and analyzed its prognostic relevance in 355 patients. FLT3-ITD of various length and load were detected in 243/638 cases (38.1%). Median EFS (19.3 vs 9.7 months, P<0.001) and median 2-year survival rate (72.0 vs 52.7%, P=0.006) was better in FLT3wt (n=212 with available follow-up data) than FLT3-ITD (n=143). A higher FLT3-ITD/wt ratio as continuous variable was correlated with a shorter EFS (P=0.028). When patients were separated into subgroups according to the FLT3-ITD mutation load, only a FLT3-ITD/wt ratio ⩾0.5 conferred an independent adverse impact on EFS and OS, and retained its prognostic significance also in multivariate analysis (P=0.009 for EFS, P=0.008 for OS). In conclusion, for risk estimation in NPM1 mutated AML not only the FLT3-ITD status, but also the FLT3-ITD load has to be taken into account. These data might contribute to clinical decision making in AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med 2005; 352: 254–266.
Falini B, Nicoletti I, Martelli MF, Mecucci C . Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood 2007; 109: 874–885.
Döhner K, Schlenk RF, Habdank M, Scholl C, Rücker FG, Corbacioglu A et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood 2005; 106: 3740–3746.
Schnittger S, Schoch C, Kern W, Mecucci C, Tschulik C, Martelli MF et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood 2005; 106: 3733–3739.
Verhaak RG, Goudswaard CS, van Putten W, Bijl MA, Sanders MA, Hugens W et al. Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood 2005; 106: 3747–3754.
Swerdlow S, Campo E, Lee Harris N, Jaffe E, Pileri S, Stein H et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th edn. IARC press: Lyon, 2008.
Falini B, Martelli MP, Bolli N, Sportoletti P, Liso A, Tiacci E et al. Acute myeloid leukemia with mutated nucleophosmin (NPM1): Is it a distinct entity? Blood 2011; 117: 1109–1120.
Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 1996; 10: 1911–1918.
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 2001; 98: 1752–1759.
Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood 2002; 100: 59–66.
Hayakawa F, Towatari M, Kiyoi H, Tanimoto M, Kitamura T, Saito H et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene 2000; 19: 624–631.
Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Müller C et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood 2000; 96: 3907–3914.
Seedhouse CH, Pallis M, Grundy M, Shang S, Russell NH . FLT3-ITD expression levels and their effect on STAT5 in AML with and without NPM mutations. Br J Haematol 2009; 147: 653–661.
Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res 2001; 61: 7233–7239.
Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002; 99: 4326–4335.
Gale RE, Green C, Allen C, Mead AJ, Burnett AK, Hills RK et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood 2008; 111: 2776–2784.
Schnittger S, Kern W, Tschulik C, Weiss T, Dicker F, Falini B et al. Minimal residual disease levels assessed by NPM1 mutation specific RQ-PCR provide important prognostic information in AML. Blood 2009; 114: 2220–2231.
Löffler H, Raststetter J, Haferlach T . Atlas of Clinical Hematology, 6th (edn). Springer: Berlin, 2004.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med 1985; 103: 620–625.
Schoch C, Schnittger S, Bursch S, Gerstner D, Hochhaus A, Berger U et al. Comparison of chromosome banding analysis, interphase- and hypermetaphase-FISH, qualitative and quantitative PCR for diagnosis and for follow-up in chronic myeloid leukemia: a study on 350 cases. Leukemia 2002; 16: 53–59.
Kern W, Voskova D, Schoch C, Hiddemann W, Schnittger S, Haferlach T . Determination of relapse risk based on assessment of minimal residual disease during complete remission by multiparameter flow cytometry in unselected patients with acute myeloid leukemia. Blood 2004; 104: 3078–3085.
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2003; 21: 4642–4649.
Thiede C, Koch S, Creutzig E, Steudel C, Illmer T, Schaich M et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML). Blood 2006; 107: 4011–4020.
Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med 2008; 358: 1909–1918.
Schnittger S, Schoch C, Kern W, Hiddemann W, Haferlach T . FLT3 length mutations as marker for follow-up studies in acute myeloid leukaemia. Acta Haematol 2004; 112: 68–78.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SS, WK, CH and TH declare part ownership of the MLL Munich Leukemia Laboratory. TA is employed by the MLL Munich Leukemia Laboratory. UB has nothing to disclose.
Author contributions
SS did molecular analysis, performed data analysis and contributed to writing of manuscript. UB performed data analysis and wrote manuscript. WK was responsible for immunophenotyping and, together with TA, performed statistics. CH was responsible for cytogenetics. TH performed cytomorphology and was responsible for study design. All authors contributed to writing of the manuscript and approved the final version.
Rights and permissions
About this article
Cite this article
Schnittger, S., Bacher, U., Kern, W. et al. Prognostic impact of FLT3-ITD load in NPM1 mutated acute myeloid leukemia. Leukemia 25, 1297–1304 (2011). https://doi.org/10.1038/leu.2011.97
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2011.97
Keywords
This article is cited by
-
AML with germline DDX41 variants is a clinicopathologically distinct entity with an indolent clinical course and favorable outcome
Leukemia (2022)
-
Adverse impact of a high allelic burden FLT3-ITD mutation on allogeneic hematopoietic stem cell transplantation in patients with cytogenetically normal AML
International Journal of Hematology (2022)
-
FLT3-targeted treatment for acute myeloid leukemia
International Journal of Hematology (2022)
-
Distinct clinico-biological features in AML patients with low allelic ratio FLT3-ITD: role of allogeneic stem cell transplantation in first remission
Bone Marrow Transplantation (2022)
-
NPM1-mutation-based measurable residual disease assessment after completion of two courses of post-remission therapy is a valuable clinical predictor of the prognosis of acute myeloid leukemia
International Journal of Hematology (2022)