Abstract
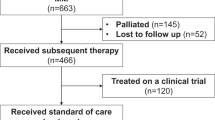
Promising new drugs are being evaluated for treatment of multiple myeloma (MM), but their impact should be measured against the expected outcome in patients failing current therapies. However, the natural history of relapsed disease in the current era remains unclear. We studied 286 patients with relapsed MM, who were refractory to bortezomib and were relapsed following, refractory to or ineligible to receive, an IMiD (immunomodulatory drug), had measurable disease, and ECOG PS of 0, 1 or 2. The date patients satisfied the entry criteria was defined as time zero (T0). The median age at diagnosis was 58 years, and time from diagnosis to T0 was 3.3 years. Following T0, 213 (74%) patients had a treatment recorded with one or more regimens (median=1; range 0–8). The first regimen contained bortezomib in 55 (26%) patients and an IMiD in 70 (33%). A minor response or better was seen to at least one therapy after T0 in 94 patients (44%) including ⩾partial response in 69 (32%). The median overall survival and event-free survival from T0 were 9 and 5 months, respectively. This study confirms the poor outcome, once patients become refractory to current treatments. The results provide context for interpreting ongoing trials of new drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rajkumar SV, Blood E, Vesole DH, Fonseca R, Greipp PR . Phase iii clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol 2006; 24: 431–436.
Rajkumar SV, Hayman S, Gertz MA, Dispenzieri A, Lacy MQ, Greipp PR et al. Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. J Clin Oncol 2002; 20: 4319–4323.
Rajkumar SV, Hayman SR, Lacy MQ, Dispenzieri A, Geyer SM, Kabat B et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood 2005; 106: 4050–4053.
Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 2005; 352: 2487–2498.
Lacy MQ, Hayman SR, Gertz MA, Dispenzieri A, Buadi F, Kumar S et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol 2009; 27: 5008–5014.
Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med 2007; 357: 2133–2142.
Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 2007; 357: 2123–2132.
Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol 2010; 11: 29–37.
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008; 111: 2516–2520.
Brenner H, Gondos A, Pulte D . Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood 2008; 111: 2521–2526.
Badros A, Burger AM, Philip S, Niesvizky R, Kolla SS, Goloubeva O et al. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin Cancer Res 2009; 15: 5250–5257.
Kumar SK, Mikhael JR, Buadi FK, Dingli D, Dispenzieri A, Fonseca R et al. Management of newly diagnosed symptomatic multiple myeloma: updated mayo stratification of myeloma and risk-adapted therapy (mSMART) consensus guidelines. Mayo Clin Proc 2009; 84: 1095–1110.
Kumar SK, Therneau TM, Gertz MA, Lacy MQ, Dispenzieri A, Rajkumar SV et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc 2004; 79: 867–874.
Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 2003; 348: 2609–2617.
Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 1999; 341: 1565–1571.
Anderson KC, Kyle RA, Rajkumar SV, Stewart AK, Weber D, Richardson P . Clinically relevant end points and new drug approvals for myeloma. Leukemia 2008; 22: 231–239.
Davis S, Wright PW, Schulman SF, Hill LD, Pinkham RD, Johnson LP et al. Participants in prospective, randomized clinical trials for resected non-small cell lung cancer have improved survival compared with nonparticipants in such trials. Cancer 1985; 56: 1710–1718.
Karjalainen S, Palva I . Do treatment protocols improve end results? A study of survival of patients with multiple myeloma in Finland. BMJ 1989; 299: 1069–1072.
Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol 1998; 102: 1115–1123.
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20: 1467–1473.
Kumar S, Lacy MQ, Dispenzieri A, Buadi F, Hayman SR, Dingli D et al. Novel Agents for Initial Therapy of Multiple Myeloma: Comparable Results with Continued Initial Therapy and Delayed Transplantation at Relapse Versus Early Transplantation. ASH Ann Meet Abstr 2009; 114: 956.
Sood R, Carloss H, Kerr R, Lopez J, Lee M, Druck M et al. Retreatment with bortezomib alone or in combination for patients with multiple myeloma following an initial response to bortezomib. Am J Hematol 2009; 84: 657–660.
Conner TM, Doan QD, Walters IB, LeBlanc AL, Beveridge RA . An observational, retrospective analysis of retreatment with bortezomib for multiple myeloma. Clin Lymph Myeloma 2008; 8: 140–145.
Warzocha K, Kraj M, Poglod R, Kwasniak B . Bortezomib in multiple myeloma: treatment and retreatment. A single center experience. Acta Pol Pharm 2008; 65: 753–756.
Wolf J, Richardson PG, Schuster M, LeBlanc A, Walters IB, Battleman DS . Utility of bortezomib retreatment in relapsed or refractory multiple myeloma patients: a multicenter case series. Clin Adv Hematol Oncol 2008; 6: 755–760.
Myeloma Trialists’ Collaborative Group. Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. J Clin Oncol 1998; 16: 3832–3842.
Gertz MA, Kumar S, Lacy MQ, Dispenzieri A, Dingli D, Hayman SR et al. Stem cell transplantation in multiple myeloma: impact of response failure with thalidomide or lenalidomide induction. Blood 2010; 115: 2348–2353.
Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J et al. International staging system for multiple myeloma. J Clin Oncol 2005; 23: 3412–3420.
Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia 2009; 23: 2210–2221.
Fonseca R, Blood E, Rue M, Harrington D, Oken MM, Kyle RA et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood 2003; 101: 4569–4575.
Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood 2007; 109: 3489–3495.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
JJL is on the Scientific advisory boards of Celgene and Janssen-Cilag. PGR and JSM are Advisory board participants for Celgene, Millenium, Johnson and Johnson. DS is on the Speakers Bureau and BD is an Advisory board participant for Celgene and Millenium. AP is an Advisory Board participant for Celgene, Johnson and Johnson. JB has received Honoraria for lectures and advisory boards from Celgene, Jansen Cilag and Grant support from Celgene and Jansen-Cilag. The remaining authors declare no conflict of interest.
Additional information
Niels Abildgaard, Syddansk Universitet, Odense, Denmark Rafat Abonour, Indiana University School of Medicine, Indianapolis, Indiana, USA Ray Alexanian, MD Anderson, Houston, Texas, USA Melissa Alsina, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA Kenneth C. Anderson, DFCI, Boston, Massachusetts, USA Michael Attal, Purpan Hospital, Toulouse, France Hervé Avet-Loiseau, Institute de Biologie, Nantes, France Ashraf Badros, University of Maryland, Baltimore, Maryland, USA Dalsu Baris, National Cancer Institute, Bethesda, Maryland, USA Bart Barlogie, M.I.R.T. UAMS Little Rock, Arkanas, USA Régis Bataille, Institute de Biologie, Nantes, France Meral Beksaç, Ankara University, Ankara, Turkey Andrew Belch, Cross Cancer Institute, Alberta, Canada Dina Ben-Yehuda, Hadassah University Hospital, Hadassah, Israel Bill Bensinger, Fred Hutchinson Cancer Center, Seattle, Washington, USA P. Leif Bergsagel, Mayo Clinic Scottsdale, Scottsdale, Arizona, USA Jenny Bird, Bristol Haematology and Oncology Center, Bristol, UK Joan Bladé, Hospital Clinica, Barcelona, Spain Mario Boccadoro, University of Torino, Torino, Italy Michele Cavo, Universita di Bologna, Bologna, Italy Asher Chanan-Khan, Roswell Park Cancer Institute, Buffalo, New York USA Wen Ming Chen, MM Research Center of Beijing, Beijing, China Tony Child, Leeds General Hospital, Leeds, United Kingdom James Chim, Department of Medicine, Queen Mary Hospital, Hong Kong Wee-Joo Chng, National University Health System, Singapore Ray Comenzo, Tufts Medical School, Boston, Massachusetts, USA John Crowley, Cancer Research and Biostatistics, Seattle, Washington, USA William Dalton, H. Lee Moffitt, Tampa, Florida, USA Faith Davies, Royal Marsden Hospital, London, England Cármino de Souza, Univeridade de Campinas, Caminas, Brazil Michel Delforge, University Hospital Gasthuisberg, Leuven, Belgium Meletios Dimopoulos, University of Athens School of Medicine, Athens, Greece Angela Dispenzieri, Mayo Clinic, Rochester, Minnesota, USA Johannes Drach, University of Vienna, Vienna, Austria Matthew Drake, Mayo Clinic Rochester, Rochester, Minnesota, USA Brian G.M. Durie, Cedars-Sinai Samuel Oschin Cancer Center, Los Angeles, California, USA Hermann Einsele, Universitätsklinik Würzburg, Würzburg, Germany Theirry Facon, Centre Hospitalier Regional Universitaire de Lille, Lille, France Dorotea Fantl, Socieded Argentinade Hematolgia, Buenos Aires, Argentina Jean-Paul Fermand, Hopitaux de Paris, Paris, France Rafael Fonseca, Mayo Clinic Arizona, Scottsdale, Arizona, USA Gösta Gahrton, Karolinska Institute for Medicine, Huddinge, Sweden Ramón García-Sanz, University Hospital of Salamanca, Salamanca, Spain Christina Gasparetto, Duke University Medical Center, Durham, North Carolina, USA Morie Gertz, Mayo Clinic, Rochester, Minnesota, USA John Gibson, Royal Prince Alfred Hospital, Sydney, Australia Sergio Giralt, MD Anderson Cancer Center, Houston, Texas, USA Hartmut Goldschmidt, University Hospital Heidelberg, Heidelberg, Germany Philip Greipp, Mayo Clinic, Rochester, Minnesota, USA Roman Hajek, Brno University, Brno, Czech Republic Izhar Hardan, Tel Aviv University, Tel Aviv, Israel Parameswaran Hari, Medical College of Wisconsin, Milwaukee, Wisconsin, USA Jean-Luc Harousseau, Institute de Biologie, Nantes, France Hiroyuki Hata, Kumamoto University Hospital, Kumamoto, Japan Yutaka Hattori, Keio University School of Medicine, Tokyo, Japan Tom Heffner, Emory University, Atlanta, Georgia, USA Joy Ho, Royal Prince Alfred Hospital, Sydney, Australia Vania Hungria, Clinica San Germano, Sao Paolo, Brazil Shinsuke Ida, Nagoya City University Medical School, Nagoya, Japan Peter Jacobs, Constantiaberg Medi-Clinic, Plumstead, South Africa Sundar Jagannath, Mt. Sinai Cancer Institute, New York, New York, USA Hans E Johnsen, AHSIC Aarhus University, Aalbor, Denmark Hou Jian, Shanghai Chang Zheng Hospital, Shanghai, China Douglas Joshua, Royal Prince Alfred Hospital, Sydney, Australia Artur Jurczyszyn, The Myeloma Treatment Foundation, Poland Michio Kawano, Yamaguchi University, Ube, Japan Nicolaus Kröger, University Hospital Hamburg, Hamburg, Germany Shaji Kumar, Department of Hematology, Mayo Clinic, Minnesota, USA Robert A. Kyle, Department of Laboratory Med. and Pathology, Mayo Clinic, Minnesota, USA Martha Lacy, Mayo Clinic Rochester, Rochester, Minnesota, USA Juan José Lahuerta, Grupo Español di Mieloma, Hospital Universitario 12 de Octubre, Madrid, Spain Ola Landgren, National Cancer Institute, Bethesda, Maryland, USA Jacob Laubach, Dana-Farber Cancer Institute, Boston, Massachusetts, USA Jae Hoon Lee, Gachon University Gil Hospital, Incheon, Korea Xavier LeLeu, Hospital Huriez, CHRU Lille, France Suzanne Lentzsch, University of Pittsburgh, Pittsburgh, Pennsylvania, USA Henk Lokhorst, University Medical CenterUtrecht, Utrecht, The Netherlands Sagar Lonial, Emory University Medical School, Atlanta, Georgia, USA Heinz Ludwig, Wilhelminenspital Der Stat Wien, Vienna, Austria Angelo Maiolino, Rua fonte da Saudade, Rio de Janeiro, Brazil María Mateos, University of Salamanca, Salamanca, Spain Jayesh Mehta, Northwestern University, Chicago, Illinois, USA Ulf-Henrik Mellqvist, Sahlgrenska University Hospital, Gothenburg, Sweden GiamPaolo Merlini, University of Pavia, Pavia, Italy Joseph Mikhael, Mayo Clinic Arizona, Scottsdale, Arizona, USA Angelina Rodríguez Morales, Bonco Metro Politano de Sangre, Caracas, Venezuela Philippe Moreau, University Hospital, Nantes, France Gareth Morgan, Royal Marsden Hospital, London, England Hareth Nari, Karolinska University Hospital, Stockholm, Sweden Nikhil Munshi, Diane Farber Cancer Institute, Boston, Massachusetts, USA Ruben Niesvizky, Weill Medical College of Cornell University, New York, New York, USA Amara Nouel, Hospital Rutz y Paez, Bolivar, Venezuela Yana Novis, Hospital SírioLibanês, Bela Vista, Brazil Robert Orlowski, MD Anderson Cancer Center, Houston, Texas, USA Antonio Palumbo, Cathedra Ematologia, Torino, Italy Santiago Pavlovsky, Fundaleu, Buenos Aires, Argentina Linda Pilarski, University of Alberta, Alberta, Canada Raymond Powles, Leukemia & Myeloma, Wimbledon, England Noopur Raje, Massachusetts General Hospital, Boston, Massachusetts, USA S. Vincent Rajkumar, Mayo Clinic, Rochester, Minnesota, USA Donna Reece, Princess Margaret Hospital, Toronto, Canada Tony Reiman, Cross Cancer Institute, Alberta, Canada Paul G. Richardson, Dana Farber Cancer Institute, Boston, Massachusetts, USA David Roodman, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania USA Laura Rosiñol, Hospital Clinic, Barcelona, Spain Jesús San Miguel, University of Salamanca, Salamanca, Spain Orhan Sezer, Universität Hamburg, Hamburg, Germany Jatin J. Shah, MD Anderson Cancer Institute, Houston, Texas, USA John Shaughnessy, M.I.R.T. UAMS, Little Rock, Arkansas, USA Kazuyuki Shimizu, Nagoya City Midori General Hospital, Nagoya, Japan Chaim Shustik, McGill University, Montreal, Canada David Siegel, Hackensack, Cancer Center, Hackensack, New Jersey, USA Seema Singhal, Northwestern University, Chicago, Illinois, USA Pieter Sonneveld, Erasmus MC, Rotterdam, The Netherlands Andrew Spencer, The Alfred Hospital, Melbourne, Australia Edward Stadtmauer, University of Pennsylvania, Philadelphia, Pennsylvania, USA Keith Stewart, Mayo Clinic Arizona, Scottsdale, Arizona, USA Evangelos Terpos, University of Athens School of Medicine, Athens, Greece Patrizia Tosi, Italian Cooperative Group, Istituto di Ematologia Seragnoli, Bologna, Italy Guido Tricot, Huntsman Cancer Institute, Salt Lake City, Utah, USA Ingemar Turesson, SKANE University Hospital, Malmo, Sweden Ben Van Camp, Vrije Universiteit Brussels, Brussels, Belgium Brian Van Ness, University of Minnesota, Minneapolis, Minnesota, USA Ivan Van Riet, Brussels Vrija University, Brussels, Belgium Isabelle Vande Broek, Vrije Universiteit Brussels, Brussels, Belgium Karin Vanderkerken, Vrije University Brussels VUB, Brussels, Belgium Robert Vescio, Cedars-Sinai Cancer Center, Los Angeles, California, USA David Vesole, Hackensack Cancer Center, Hackensack, New Jersey, USA Anders Waage, University Hospital, Trondheim, Norway NSMG Michael Wang, MD Anderson, Houston, Texas, USA Donna Weber, MD Anderson, Houston, Texas, USA Jan Westin, Sahlgrenska University Hospital, Gothenburg, Sweden Keith Wheatley, University of Birmingham, Birmingham, United Kingdom Jeffrey Zonder, Karmanos Cancer Institute, Detroit, Michigan, USA
Author’s contributions
All authors (except JC, JH, JF and AH) provided patient data and were involved in manuscript preparation. JC, JH, JF and AH were involved in the statistical analysis.
Supplementary Information accompanies the paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Kumar, S., Lee, J., Lahuerta, J. et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia 26, 149–157 (2012). https://doi.org/10.1038/leu.2011.196
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2011.196
Keywords
This article is cited by
-
Efficacy and safety of generic pomalidomide plus low-dose dexamethasone in relapsed or refractory multiple myeloma: a multicenter, open-label, single-arm trial
Annals of Hematology (2024)
-
Immune checkpoint inhibitors for multiple myeloma immunotherapy
Experimental Hematology & Oncology (2023)
-
Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: phase 1 UNIVERSAL trial interim results
Nature Medicine (2023)
-
ML-based sequential analysis to assist selection between VMP and RD for newly diagnosed multiple myeloma
npj Precision Oncology (2023)
-
Inhibition of CARM1 suppresses proliferation of multiple myeloma cells through activation of p53 signaling pathway
Molecular Biology Reports (2023)