Abstract
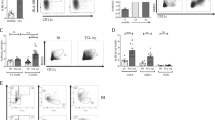
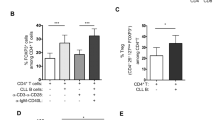
Malignant B lymphocytes from chronic lymphocytic leukemia (CLL) patients maintain the capacity to respond to CD40 ligation, among other microenvironmental stimuli. In this study, we show that (i) leukemic CLL cells stimulated with the soluble form of CD40L in vitro show differential responses in terms of upregulation of surface markers (CD95 and CD80) and induction of chemokines (CCL22 and CCL17) expression/secretion, and that (ii) these changes are mirrored by a distinct activation of intracellular signalling pathways including increase in IKKalpha/beta phosphorylation and upregulation of antiapoptotic proteins (BCL-2 and MCL-1). CLL patients can then be segregated into two distinct functional subsets. We defined the responsive subset of cases CD40L dependent, considering the capacity to respond as a sign of persistent need of this stimulation for the leukemic expansion. Conversely, we named the unresponsive cases CD40L independent, considering them less dependent on this microenvironmental signal, presumably because of a higher autonomous proliferative and survival potential. Importantly, we report that (iii) the two functional subsets show an opposite clinical outcome, with CD40L-independent cases having a shorter time to progression. This indicates that the functional differences observed in vitro may reflect a different leukemic potential in vivo likely responsible for a distinct clinical course.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
09 November 2011
This article has been corrected since advance Online Publication, and a corrigendum is also printed in this issue
References
Ghia P, Granziero L, Chilosi M, Caligaris-Cappio F . Chronic B cell malignancies and bone marrow microenvironment. Sem Cancer Biol 2002; 12: 149–155.
Caligaris-Cappio F, Ghia P . Novel insights in chronic lymphocytic leukemia: are we getting closer to understanding the pathogenesis of the disease? J Clin Oncol 2008; 26: 4497–4503.
Stevenson FK, Caligaris-Cappio F . Chronic lymphocytic leukemia: revelations from the B-cell receptor. Blood 2004; 103: 4389–4395.
Ghia P, Chiorazzi N, Stamatopoulos K . Microenvironmental influences in chronic lymphocytic leukaemia: the role of antigen stimulation. J Intern Med 2008; 264: 549–562.
Lanham S, Hamblin T, Oscier D, Ibbotson R, Stevenson F, Packham G . Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood 2003; 101: 1087–1093.
Chen L, Widhopf G, Huynh L, Rassenti L, Rai KR, Weiss A et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood 2002; 100: 4609–4614.
Muzio M, Apollonio B, Scielzo C, Frenquelli M, Vandoni I, Boussiotis V et al. Constitutive activation of distinct BCR-signaling pathways in a subset of CLL patients: a molecular signature of anergy. Blood 2008; 112: 188–195.
Zupo S, Isnardi L, Megna M, Massara R, Malavasi F, Dono M et al. CD38 expression distinguishes two groups of B-cell chronic lymphocytic leukemias with different responses to anti-IgM antibodies and propensity to apoptosis. Blood 1996; 88: 1365–1374.
Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med 2003; 348: 1764–1775.
Wiestner A, Rosenwald A, Barry TS, Wright G, Davis RE, Henrickson SE et al. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood 2003; 101: 4944–4951.
Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan IC . Mechanism of antigen-driven selection in germinal centres. Nature 1989; 342: 929–931.
Stamenkovic I, Clark EA, Seed B . A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J 1989; 8: 1403–1410.
van Kooten C, Banchereau J . Functions of CD40 on B cells, dendritic cells and other cells. Curr Opin Immunol 1997; 9: 330–337.
Kehry MR . CD40-mediated signaling in B cells. Balancing cell survival, growth, and death. J Immunol 1996; 156: 2345–2348.
Buske C, Gogowski G, Schreiber K, Rave-Frank M, Hiddemann W, Wormann B . Stimulation of B-chronic lymphocytic leukemia cells by murine fibroblasts, IL-4, anti-CD40 antibodies, and the soluble CD40 ligand. Exp Hematol 1997; 25: 329–337.
Furman RR, Asgary Z, Mascarenhas JO, Liou HC, Schattner EJ . Modulation of NF-kappa B activity and apoptosis in chronic lymphocytic leukemia B cells. J Immunol 2000; 164: 2200–2206.
Granziero L, Circosta P, Scielzo C, Frisaldi E, Stella S, Geuna M et al. CD100/Plexin-B1 interactions sustain proliferation and survival of normal and leukemic CD5+ B lymphocytes. Blood 2003; 101: 1962–1969.
Granziero L, Ghia P, Circosta P, Gottardi D, Strola G, Geuna M et al. Survivin is expressed on CD40 stimulation and interfaces proliferation and apoptosis in B-cell chronic lymphocytic leukemia. Blood 2001; 97: 2777–2783.
Wang D, Freeman GJ, Levine H, Ritz J, Robertson MJ . Role of the CD40 and CD95 (APO-1/Fas) antigens in the apoptosis of human B-cell malignancies. Br J Haematol 1997; 97: 409–417.
Ranheim EA, Kipps TJ . Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med 1993; 177: 925–935.
Kitada S, Zapata JM, Andreeff M, Reed JC . Bryostatin and CD40-ligand enhance apoptosis resistance and induce expression of cell survival genes in B-cell chronic lymphocytic leukaemia. Br J Haematol 1999; 106: 995–1004.
Willimott S, Baou M, Naresh K, Wagner SD . CD154 induces a switch in pro-survival Bcl-2 family members in chronic lymphocytic leukaemia. Br J Haematol 2007; 138: 721–732.
Ghia P, Transidico P, Veiga JP, Schaniel C, Sallusto F, Matsushima K et al. Chemoattractants MDC and TARC are secreted by malignant B-cell precursors following CD40 ligation and support the migration of leukemia-specific T cells. Blood 2001; 98: 533–540.
Pizzolo G, Chilosi M, Ambrosetti A, Semenzato G, Fiore-Donati L, Perona G . Immunohistologic study of bone marrow involvement in B-chronic lymphocytic leukemia. Blood 1983; 62: 1289–1296.
Caligaris-Cappio F . Role of the microenvironment in chronic lymphocytic leukaemia. Br J Haematol 2003; 123: 380–388.
Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ . Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009; 229: 152–172.
Muzio M, Scielzo C, Bertilaccio MT, Frenquelli M, Ghia P, Caligaris-Cappio F . Expression and function of toll like receptors in chronic lymphocytic leukaemia cells. Br J Haematol 2009; 144: 507–516.
Kato K, Cantwell MJ, Sharma S, Kipps TJ . Gene transfer of CD40-ligand induces autologous immune recognition of chronic lymphocytic leukemia B cells. J Clin Invest 1998; 101: 1133–1141.
Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F . The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood 2009; 114: 3367–3375.
Wierda WG, Cantwell MJ, Woods SJ, Rassenti LZ, Prussak CE, Kipps TJ . CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood 2000; 96: 2917–2924.
Luqman M, Klabunde S, Lin K, Georgakis GV, Cherukuri A, Holash J et al. The antileukemia activity of a human anti-CD40 antagonist antibody, HCD122, on human chronic lymphocytic leukemia cells. Blood 2008; 112: 711–720.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111: 5446–5456.
Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 1981; 48: 198–206.
Rai KR, Han T . Prognostic factors and clinical staging in chronic lymphocytic leukemia. Hematol/Oncol Clin N Am 1990; 4: 447–456.
Ghia P, Guida G, Stella S, Gottardi D, Geuna M, Strola G et al. The pattern of CD38 expression defines a distinct subset of chronic lymphocytic leukemia (CLL) patients at risk of disease progression. Blood 2003; 101: 1262–1269.
Ghia P, Stamatopoulos K, Belessi C, Moreno C, Stilgenbauer S, Stevenson F et al. ERIC recommendations on IGHV gene mutational status analysis in chronic lymphocytic leukemia. Leukemia 2007; 21: 1–3.
Fazi C, Dagklis A, Cottini F, Scarfo L, Bertilaccio MT, Finazzi R et al. Monoclonal B cell lymphocytosis in hepatitis C virus infected individuals. Cytometry 2010; 78 (Suppl 1): S61–S68.
Scielzo C, Camporeale A, Geuna M, Alessio M, Poggi A, Zocchi MR et al. ZAP-70 is expressed by normal and malignant human B-cell subsets of different maturational stage. Leukemia 2006; 20: 689–695.
Ghia P, Strola G, Granziero L, Geuna M, Guida G, Sallusto F et al. Chronic lymphocytic leukemia B cells are endowed with the capacity to attract CD4+, CD40L+ T cells by producing CCL22. Eur J Immunol 2002; 32: 1403–1413.
Stacchini A, Aragno M, Vallario A, Alfarano A, Circosta P, Gottardi D et al. MEC1 and MEC2: two new cell lines derived from B-chronic lymphocytic leukaemia in prolymphocytoid transformation. Leukemia Res 1999; 23: 127–136.
Battle TE, Wierda WG, Rassenti LZ, Zahrieh D, Neuberg D, Kipps TJ et al. In vivo activation of signal transducer and activator of transcription 1 after CD154 gene therapy for chronic lymphocytic leukemia is associated with clinical and immunologic response. Clin Cancer Res 2003; 9: 2166–2172.
Tromp JM, Tonino SH, Elias JA, Jaspers A, Luijks DM, Kater AP et al. Dichotomy in NF-kappaB signaling and chemoresistance in immunoglobulin variable heavy-chain-mutated versus unmutated CLL cells upon CD40/TLR9 triggering. Oncogene 2010; 29: 5071–5082.
Wierda WG, Castro JE, Aguillon R, Sampath D, Jalayer A, McMannis J et al. A phase I study of immune gene therapy for patients with CLL using a membrane-stable, humanized CD154. Leukemia 2010; 24: 1893–1900.
Ghia P, Circosta P, Scielzo C, Vallario A, Camporeale A, Granziero L et al. Differential effects on CLL cell survival exerted by different microenvironmental elements. Curr Top Microbiol Immunol 2005; 294: 135–145.
Plander M, Seegers S, Ugocsai P, Diermeier-Daucher S, Ivanyi J, Schmitz G et al. Different proliferative and survival capacity of CLL-cells in a newly established in vitro model for pseudofollicles. Leukemia 2009; 23: 2118–2128.
Longo PG, Laurenti L, Gobessi S, Petlickovski A, Pelosi M, Chiusolo P et al. The Akt signaling pathway determines the different proliferative capacity of chronic lymphocytic leukemia B-cells from patients with progressive and stable disease. Leukemia 2007; 21: 110–120.
Tarnani M, Laurenti L, Longo PG, Piccirillo N, Gobessi S, Mannocci A et al. The proliferative response to CpG-ODN stimulation predicts PFS, TTT and OS in patients with chronic lymphocytic leukemia. Leukemia Res 2010; 34: 1189–1194.
Lanzavecchia A, Sallusto F . Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr Opin Immunol 2007; 19: 268–274.
Acknowledgements
We thank Luisa Granziero, Paola Circosta, Massimo Geuna and Giuseppe Guida for helpful suggestions and technical support. This project was supported by: Associazione Italiana per la Ricerca sul Cancro AIRC (Investigator Grant and Special Program Molecular Clinical Oncology—5 per mille #9965), ‘CLLGRF—U.S./European Alliance for the Therapy of CLL’, FIRB and PRIN—Ministero Istruzione, Università e Ricerca (MIUR), Roma, Progetti Integrati Oncologia (PIO)—Ministero della Salute, Roma. CS is supported by the EHA Fellowship Program (2009/18), AJ is supported by a EHA Partner Fellowship Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This article has been corrected since advance Online Publication, and a corrigendum is also printed in this issue
Rights and permissions
About this article
Cite this article
Scielzo, C., Apollonio, B., Scarfò, L. et al. The functional in vitro response to CD40 ligation reflects a different clinical outcome in patients with chronic lymphocytic leukemia. Leukemia 25, 1760–1767 (2011). https://doi.org/10.1038/leu.2011.149
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2011.149
Keywords
This article is cited by
-
Targeting the proliferative and chemoresistant compartment in chronic lymphocytic leukemia by inhibiting survivin protein
Leukemia (2014)
-
“Role of the B-cell receptor and the microenvironment in chronic lymphocytic leukemia’’
Blood Cancer Journal (2013)
-
Stromal cells and CD40 ligand (CD154) alter the miRNome and induce miRNA clusters including, miR-125b/miR-99a/let-7c and miR-17-92 in chronic lymphocytic leukaemia
Leukemia (2012)
-
Immune Reconstitution in Chronic Lymphocytic Leukemia
Current Hematologic Malignancy Reports (2012)