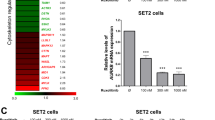
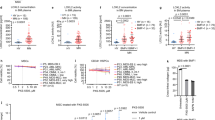
Abstract
The association between azacitidine (AZA) and valproic acid (VPA) has shown high response rates in high-risk myelodysplastic syndromes (MDS) cases with unfavorable prognosis. However, little is known about the molecular mechanisms underlying this therapy, and molecular markers useful to monitor the disease and the effect of the treatment are needed. Phosphoinositide-phospholipase C (PI-PLC) β1 is involved in both genetic and epigenetic mechanisms of MDS progression to acute myeloid leukemia. Indeed, AZA as a single agent was able to induce PI-PLCβ1 expression, therefore providing a promising new tool in the evaluation of response to demethylating therapies. In this study, we assessed the efficacy of the combination of AZA and VPA on inducing PI-PLCβ1 expression in high-risk MDS patients. Furthermore, we observed an increase in Cyclin D3 expression, a downstream target of PI-PLCβ1 signaling, therefore suggesting a potential combined activity of AZA and VPA in high-risk MDS in activating PI-PLCβ1 signaling, thus affecting cell proliferation and differentiation. Taken together, our findings might open up new lines of investigations aiming at evaluating the role of the activation of PI-PLCβ1 signaling in the epigenetic therapy, which may also lead to the identification of innovative targets for the epigenetic therapy of high-risk MDS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sebova K, Fridrichova I . Epigenetic tools in potential anticancer therapy. Anticancer Drugs 2010; 21: 565–577.
Issa JP . Epigenetic changes in the myelodysplastic syndrome. Hematol Oncol Clin North Am 2010; 24: 317–330.
Guo Y, Engelhardt M, Wider D, Abdelkarim M, Lubbert M . Effects of 5-aza-2′-deoxycytidine on proliferation, differentiation and p15/INK4b regulation of human hematopoietic progenitor cells. Leukemia 2006; 20: 115–121.
Berg T, Guo Y, Abdelkarim M, Fliegauf M, Lubbert M . Reversal of p15/INK4b hypermethylation in AML1/ETO-positive and -negative myeloid leukemia cell lines. Leuk Res 2007; 31: 497–506.
Koschmieder S, Agrawal S, Radomska HS, Huettner CS, Tenen DG, Ottmann OG et al. Decitabine and vitamin D3 differentially affect hematopoietic transcription factors to induce monocytic differentiation. Int J Oncol 2007; 30: 349–355.
Daurkin I, Eruslanov E, Vieweg J, Kusmartsev S . Generation of antigen-presenting cells from tumor-infiltrated CD11b myeloid cells with DNA demethylating agent 5-aza-2′-deoxycytidine. Cancer Immunol Immunother 2010; 59: 697–706.
Mahmud M, Stebbing J . Epigenetic modifications in AML and MDS. Leuk Res 2010; 34: 139–140.
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10: 223–232.
Fenaux P, Gattermann N, Seymour JF, Hellstrom-Lindberg E, Mufti GJ, Duehrsen U et al. Prolonged survival with improved tolerability in higher-risk myelodysplastic syndromes: azacitidine compared with low dose ara-C. Br J Haematol 2010; 149: 244–249.
Neff T, Armstrong SA . Chromatin maps, histone modifications and leukemia. Leukemia 2009; 23: 1243–1251.
Bhalla KN . Epigenetic and chromatin modifiers as targeted therapy of hematologic malignancies. J Clin Oncol 2005; 23: 3971–3993.
Minucci S, Pelicci PG . Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 2006; 6: 38–51.
Gore SD, Hermes-DeSantis ER . Future directions in myelodysplastic syndrome: newer agents and the role of combination approaches. Cancer Control 2008; 15 (Suppl): 40–49.
Mercurio C, Minucci S, Pelicci PG . Histone deacetylases and epigenetic therapies of hematological malignancies. Pharmacol Res 2010; 62: 18–34.
Braiteh F, Soriano AO, Garcia-Manero G, Hong D, Johnson MM, Silva Lde P et al. Phase I study of epigenetic modulation with 5-azacytidine and valproic acid in patients with advanced cancers. Clin Cancer Res 2008; 14: 6296–6301.
Voso MT, Santini V, Finelli C, Musto P, Pogliani E, Angelucci E et al. Valproic acid at therapeutic plasma levels may increase 5-azacytidine efficacy in higher risk myelodysplastic syndromes. Clin Cancer Res 2009; 15: 5002–5007.
Kuendgen A, Lubbert M . Current status of epigenetic treatment in myelodysplastic syndromes. Ann Hematol 2008; 87: 601–611.
Gore SD . In vitro basis for treatment with hypomethylating agents and histone deacetylase inhibitors: can epigenetic changes be used to monitor treatment? Leuk Res 2009; 33 (Suppl 2): S2–S6.
Silverman LR . Hypomethylating agents in myelodysplastic syndromes changing the inevitable: the value of azacitidine as maintenance therapy, effects on transfusion and combination with other agents. Leuk Res 2009; 33 (Suppl 2): S18–S21.
McCubrey JA, Steelman LS, Abrams SL, Bertrand FE, Ludwig DE, Basecke J et al. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia 2008; 22: 708–722.
Follo MY, Mongiorgi S, Finelli C, Clissa C, Ramazzotti G, Fiume R et al. Nuclear inositide signaling in myelodysplastic syndromes. J Cell Biochem 2010; 109: 1065–1071.
Faenza I, Matteucci A, Manzoli L, Billi AM, Aluigi M, Peruzzi D et al. A role for nuclear phospholipase Cbeta 1 in cell cycle control. J Biol Chem 2000; 275: 30520–30524.
O’Carroll SJ, Mitchell MD, Faenza I, Cocco L, Gilmour RS . Nuclear PLCbeta1 is required for 3T3-L1 adipocyte differentiation and regulates expression of the cyclin D3-cdk4 complex. Cell Signal 2009; 21: 926–935.
Furukawa Y . Cell cycle control genes and hematopoietic cell differentiation. Leuk Lymphoma 2002; 43: 225–231.
Cooper AB, Sawai CM, Sicinska E, Powers SE, Sicinski P, Clark MR et al. A unique function for cyclin D3 in early B cell development. Nat Immunol 2006; 7: 489–497.
Follo MY, Finelli C, Clissa C, Mongiorgi S, Bosi C, Martinelli G et al. Phosphoinositide-phospholipase C beta1 mono-allelic deletion is associated with myelodysplastic syndromes evolution into acute myeloid leukemia. J Clin Oncol 2009; 27: 782–790.
Follo MY, Finelli C, Bosi C, Martinelli G, Mongiorgi S, Baccarani M et al. PI-PLCbeta-1 and activated Akt levels are linked to azacitidine responsiveness in high-risk myelodysplastic syndromes. Leukemia 2008; 22: 198–200.
Follo MY, Finelli C, Mongiorgi S, Clissa C, Bosi C, Testoni N et al. Reduction of phosphoinositide-phospholipase C beta1 methylation predicts the responsiveness to azacitidine in high-risk MDS. Proc Natl Acad Sci USA 2009; 106: 16811–16816.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol 1982; 51: 189–199.
Vardiman JW, Harris NL, Brunning RD . The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 2002; 100: 2292–2302.
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114: 937–951.
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89: 2079–2088.
Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006; 108: 419–425.
Follo MY, Bosi C, Finelli C, Fiume R, Faenza I, Ramazzotti G et al. Real-time PCR as a tool for quantitative analysis of PI-PLCbeta1 gene expression in myelodysplastic syndrome. Int J Mol Med 2006; 18: 267–271.
Follo MY, Mongiorgi S, Bosi C, Cappellini A, Finelli C, Chiarini F et al. The Akt/mammalian target of rapamycin signal transduction pathway is activated in high-risk myelodysplastic syndromes and influences cell survival and proliferation. Cancer Res 2007; 67: 4287–4294.
Chiarini F, Del Sole M, Mongiorgi S, Gaboardi GC, Cappellini A, Mantovani I et al. The novel Akt inhibitor, perifosine, induces caspase-dependent apoptosis and downregulates P-glycoprotein expression in multidrug-resistant human T-acute leukemia cells by a JNK-dependent mechanism. Leukemia 2008; 22: 1106–1116.
Ravandi F, Issa JP, Garcia-Manero G, O’Brien S, Pierce S, Shan J et al. Superior outcome with hypomethylating therapy in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome and chromosome 5 and 7 abnormalities. Cancer 2009; 115: 5746–5751.
Seymour JF, Fenaux P, Silverman LR, Mufti GJ, Hellstrom-Lindberg E, Santini V et al. Effects of azacitidine compared with conventional care regimens in elderly (⩾75 years) patients with higher-risk myelodysplastic syndromes. Crit Rev Oncol Hematol 2010; 76: 218–227.
Abujamra AL, Dos Santos MP, Roesler R, Schwartsmann G, Brunetto AL . Histone deacetylase inhibitors: a new perspective for the treatment of leukemia. Leuk Res 2010; 34: 687–695.
Tan J, Cang S, Ma Y, Petrillo RL, Liu D . Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. J Hematol Oncol 2010; 3: 5.
Visvader J, Adams JM . Megakaryocytic differentiation induced in 416B myeloid cells by GATA-2 and GATA-3 transgenes or 5-azacytidine is tightly coupled to GATA-1 expression. Blood 1993; 82: 1493–1501.
Zinzar S, Silverman LR, Richardson EB, Bekesi G, Holland JF . Azacytidine plus verapamil induces the differentiation of a newly characterized biphenotypic human myeloid-B lymphoid leukemic cell line BW-90. Leuk Res 1998; 22: 677–685.
Gurvich N, Tsygankova OM, Meinkoth JL, Klein PS . Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res 2004; 64: 1079–1086.
Cheng YC, Lin H, Huang MJ, Chow JM, Lin S, Liu HE . Downregulation of c-Myc is critical for valproic acid-induced growth arrest and myeloid differentiation of acute myeloid leukemia. Leuk Res 2007; 31: 1403–1411.
Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Basecke J et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia 2008; 22: 686–707.
Acknowledgements
This work was supported by the Italian Ministry of Education, University and Research—Funds for Basic Research: Human Proteome Net; Italian Ministry of Education, University and Research: Research Projects of National Interest; Cassa di Risparmio in Bologna Foundation, Bologna, Italy; European Leukemia Net; Italian Association for Cancer Research; Italian Association of Leukemia; and the ‘Alfredo Saiardi Association’ for Hematological Malignancies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Follo, M., Finelli, C., Mongiorgi, S. et al. Synergistic induction of PI-PLCβ1 signaling by azacitidine and valproic acid in high-risk myelodysplastic syndromes. Leukemia 25, 271–280 (2011). https://doi.org/10.1038/leu.2010.266
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2010.266
Keywords
This article is cited by
-
Response of high-risk MDS to azacitidine and lenalidomide is impacted by baseline and acquired mutations in a cluster of three inositide-specific genes
Leukemia (2019)
-
Expression of nucleoside-metabolizing enzymes in myelodysplastic syndromes and modulation of response to azacitidine
Leukemia (2014)
-
Epigenetics in clinical practice: the examples of azacitidine and decitabine in myelodysplasia and acute myeloid leukemia
Leukemia (2013)
-
Epigenetic regulation of nuclear PI-PLCbeta1 signaling pathway in low-risk MDS patients during azacitidine treatment
Leukemia (2012)