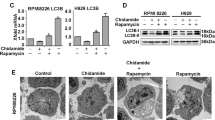
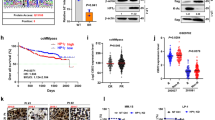
Abstract
Interactions between inhibitors of the proteasome and histone deacetylases have been examined in human T-leukemia/lymphoma cells both in vitro and in vivo. Co-exposure of cells to bortezomib and suberoylanilide hydroxamic acid (SAHA) synergistically induces T-leukemia/lymphoma cells to undergo apoptosis, consistent with a significant increase in mitochondrial injury and caspase activation. These events are accompanied by inhibition of cyto-protective signaling pathways, including the nuclear factor (NF)-κB, Raf-1/mitogen-induced extracellular kinase (MEK)/extracellular signal-related kinase (ERK) and AKT pathways, and activation of stress-related cascades, including the stress-activated kinases c-jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (p38MAPK). Moreover, bortezomib in conjunction with SAHA efficiently induces apoptosis of primary T-leukemia/lymphoma cells and inhibits tumor growth in a murine xenograft model established with subcutaneous injection of Jurkat cells. Taken together, these findings confirm the synergistic anti-tumor effect of the proteasome and histone deacetylase inhibitors, and provide an insight into the future clinical applications of bortezomib–SAHA combining regimen in treating T-cell malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hoelzer D, Gokbuget N, Ottmann O, Pui CH, Relling MV, Appelbaum FR et al. Acute lymphoblastic leukemia. Hematol Am Soc Hematol Educ Program 2002, 162–192.
Savage KJ . Aggressive peripheral T-cell lymphomas (specified and unspecified types). Hematol Am Soc Hematol Educ Program 2005, 267–277.
Vilimas T, Mascarenhas J, Palomero T, Mandal M, Buonamici S, Meng F et al. Targeting the NF-kappaB signaling pathway in Notch1-induced T-cell leukemia. Nat Med 2007; 13: 70–77.
Martinez-Delgado B, Cuadros M, Honrado E, Ruiz de la Parte A, Roncador G, Alves J et al. Differential expression of NF-kappaB pathway genes among peripheral T-cell lymphomas. Leukemia 2005; 19: 2254–2263.
Barata JT, Cardoso AA, Boussiotis VA . Interleukin-7 in T-cell acute lymphoblastic leukemia: an extrinsic factor supporting leukemogenesis? Leuk Lymphoma 2005; 46: 483–495.
Marzec M, Kasprzycka M, Liu X, El-Salem M, Halasa K, Raghunath PN et al. Oncogenic tyrosine kinase NPM/ALK induces activation of the rapamycin-sensitive mTOR signaling pathway. Oncogene 2007; 26: 5606–5614.
Vega F, Medeiros LJ, Leventaki V, Atwell C, Cho-Vega JH, Tian L et al. Activation of mammalian target of rapamycin signaling pathway contributes to tumor cell survival in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Cancer Res 2006; 66: 6589–6597.
Zhang W, McQueen T, Schober W, Rassidakis G, Andreeff M, Konopleva M . Leukotriene B4 receptor inhibitor LY293111 induces cell cycle arrest and apoptosis in human anaplastic large-cell lymphoma cells via JNK phosphorylation. Leukemia 2005; 19: 1977–1984.
Lu J, Quearry B, Harada H . p38-MAP kinase activation followed by BIM induction is essential for glucocorticoid-induced apoptosis in lymphoblastic leukemia cells. FEBS Lett 2006; 580: 3539–3544.
Orlowski RZ . The ubiquitin proteasome pathway from bench to bedside. Hematol Am Soc Hematol Educ Program 2005, 220–225.
Mai W, Meng H, Jin J, Wang L . Treatment with bortezomib in a patient with heavily pretreated refractory T-cell lymphoblastic lymphoma. Eur J Haematol 2006; 77: 445–447.
Zinzani PL, Musuraca G, Tani M, Stefoni V, Marchi E, Fina M et al. Phase II trial of proteasome inhibitor bortezomib in patients with relapsed or refractory cutaneous T-cell lymphoma. J Clin Oncol 2007; 25: 4293–4297.
Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK . Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 2001; 1: 194–202.
Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood 2007; 109: 31–39.
Pei XY, Dai Y, Grant S . Synergistic induction of oxidative injury and apoptosis in human multiple myeloma cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitors. Clin Cancer Res 2004; 10: 3839–3852.
Heider U, von Metzler I, Kaiser M, Rosche M, Sterz J, Rotzer S et al. Synergistic interaction of the histone deacetylase inhibitor SAHA with the proteasome inhibitor bortezomib in mantle cell lymphoma. Eur J Haematol 2008; 80: 133–142.
Dai Y, Chen S, Kramer LB, Funk VL, Dent P, Grant S . Interactions between Bortezomib and Romidepsin and Belinostat in chronic lymphocytic leukemia cells. Clin Cancer Res 2008; 14: 549–558.
Kano Y, Akutsu M, Tsunoda S, Mano H, Sato Y, Honma Y et al. In vitro cytotoxic effects of a tyrosine kinase inhibitor STI571 in combination with commonly used antileukemic agents. Blood 2001; 97: 1999–2007.
Zhao WL, Daneshpouy ME, Mounier N, Briere J, Leboeuf C, Plassa LF et al. Prognostic significance of bcl-xL gene expression and apoptotic cell counts in follicular lymphoma. Blood 2004; 103: 695–697.
He LZ, Tolentino T, Grayson P, Zhong S, Warrell Jr RP, Rifkind RA et al. Histone deacetylase inhibitors induce remission in transgenic models of therapy-resistant acute promyelocytic leukemia. J Clin Invest 2001; 108: 1321–1330.
Karin M, Cao Y, Greten FR, Li ZW . NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2002; 2: 301–310.
Nalepa G, Wade Harper J . Therapeutic anti-cancer targets upstream of the proteasome. Cancer Treat Rev 2003; 29 (Suppl 1): 49–57.
Lewis TS, Shapiro PS, Ahn NG . Signal transduction through MAP kinase cascades. Adv Cancer Res 1998; 74: 49–139.
Nishioka C, Ikezoe T, Yang J, Koeffler HP, Yokoyama A . Inhibition of MEK/ERK signaling synergistically potentiates histone deacetylase inhibitor-induced growth arrest, apoptosis and acetylation of histone H3 on p21(waf1) promoter in acute myelogenous leukemia cell. Leukemia 2008; 22: 1449–1452.
Yu C, Dasmahapatra G, Dent P, Grant S . Synergistic interactions between MEK1/2 and histone deacetylase inhibitors in BCR/ABL+ human leukemia cells. Leukemia 2005; 19: 1579–1589.
Kawamata N, Chen J, Koeffler HP . Suberoylanilide hydroxamic acid (SAHA; vorinostat) suppresses translation of cyclin D1 in mantle cell lymphoma cells. Blood 2007; 110: 2667–2673.
Khan T, Stauffer JK, Williams R, Hixon JA, Salcedo R, Lincoln E et al. Proteasome inhibition to maximize the apoptotic potential of cytokine therapy for murine neuroblastoma tumors. J Immunol 2006; 176: 6302–6312.
Shelton JG, Blalock WL, White ER, Steelman LS, McCubrey JA . Ability of the activated PI3K/Akt oncoproteins to synergize with MEK1 and induce cell cycle progression and abrogate the cytokine-dependence of hematopoietic cells. Cell Cycle 2004; 3: 503–512.
Cuenda A, Rousseau S . p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta 2007; 1773: 1358–1375.
Dolado I, Swat A, Ajenjo N, De Vita G, Cuadrado A, Nebreda AR . p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell 2007; 11: 191–205.
Yu C, Rahmani M, Dent P, Grant S . The hierarchical relationship between MAPK signaling and ROS generation in human leukemia cells undergoing apoptosis in response to the proteasome inhibitor Bortezomib. Exp Cell Res 2004; 295: 555–566.
Yu C, Subler M, Rahmani M, Reese E, Krystal G, Conrad D et al. Induction of apoptosis in BCR/ABL+ cells by histone deacetylase inhibitors involves reciprocal effects on the RAF/MEK/ERK and JNK pathways. Cancer Biol Ther 2003; 2: 544–551.
Yu C, Rahmani M, Conrad D, Subler M, Dent P, Grant S . The proteasome inhibitor bortezomib interacts synergistically with histone deacetylase inhibitors to induce apoptosis in Bcr/Abl+ cells sensitive and resistant to STI571. Blood 2003; 102: 3765–3774.
Acknowledgements
We thank Professor Arthur Zelent for his critical review of the paper. This work was supported, in part, by the Chinese National Key Program for Basic Research (973:2004CB518600), the Chinese National High Tech Program (863:2006AA02A301 and 863:2006AA02A405), the National Natural Science Foundation of China (30750335), the Shanghai Commission of Science and Technology (08410708800), the Shanghai Rising Star Program (05QMX1429), the Program for New Century Excellent Talents in University, the Scientific Research Foundation for the Returned Overseas Chinese Scholars, the Fok Ying Tung Education Foundation (111035), the Programme de Recherches Avancées, the Samuel Waxman Cancer Research Foundation Laboratory and by the Co-PI Program of Shanghai Rui Jin Hospital/Shanghai Jiao Tong University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, QL., Wang, L., Zhang, YW. et al. The proteasome inhibitor bortezomib interacts synergistically with the histone deacetylase inhibitor suberoylanilide hydroxamic acid to induce T-leukemia/lymphoma cells apoptosis. Leukemia 23, 1507–1514 (2009). https://doi.org/10.1038/leu.2009.41
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2009.41
Keywords
This article is cited by
-
Sirtuin1 (SIRT1) is involved in the anticancer effect of black raspberry anthocyanins in colorectal cancer
European Journal of Nutrition (2023)
-
Exosomal annexin A6 induces gemcitabine resistance by inhibiting ubiquitination and degradation of EGFR in triple-negative breast cancer
Cell Death & Disease (2021)
-
Role of HDACs in normal and malignant hematopoiesis
Molecular Cancer (2020)
-
Restoring MLL reactivates latent tumor suppression-mediated vulnerability to proteasome inhibitors
Oncogene (2020)
-
Application of an ex-vivo drug sensitivity platform towards achieving complete remission in a refractory T-cell lymphoma
Blood Cancer Journal (2020)