Abstract
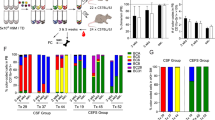
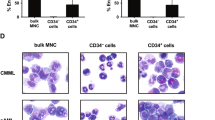
The presence of rare malignant stem cells supplying a hierarchy of malignant cells has recently been reported. In human acute myelogenous leukemia (AML), the leukemia stem cells (LSCs) have been phenotypically restricted within the CD34+CD38− fraction. To understand the origin of malignant cells in primary human B-precursor acute lymphocytic leukemia (B-ALL), we established a novel in vivo xenotransplantation model. Purified CD34+CD38+CD19+, CD34+CD38−CD19+ and CD34+CD38−CD19− bone marrow (BM) or peripheral blood (PB) cells from three pediatric B-ALL patients were intravenously injected into sublethally irradiated newborn NOD/SCID/IL2rγnull mice. We found that both CD34+CD38+CD19+ and CD34+CD38−CD19+ cells initiate B-ALL in primary recipients, whereas the recipients of CD34+CD38−CD10−CD19− cells showed normal human hematopoietic repopulation. The extent of leukemic infiltration into the spleen, liver and kidney was similar between the recipients transplanted with CD34+CD38+CD19+ cells and those transplanted with CD34+CD38−CD19+ cells. In each of the three cases studied, transplantation of CD34+CD38+CD19+ cells resulted in the development of B-ALL in secondary recipients, demonstrating self-renewal capacity. The identification of CD34+CD38+CD19+ self-renewing B-ALL cells proposes a hierarchy of leukemia-initiating cells (LICs) distinct from that of AML. Recapitulation of patient B-ALL in NOD/SCID/IL2rγnull recipients provides a powerful tool for directly studying leukemogenesis and for developing therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pui CH, Evans WE . Acute lymphoblastic leukemia. N Engl J Med 1998; 339: 605–615.
Pui CH, Evans WE . Treatment of acute lymphoblastic leukemia. N Engl J Med 2006; 354: 166–178.
O’Brien CA, Pollett A, Gallinger S, Dick JE . A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007; 445: 106–110.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T et al. Identification of human brain tumour initiating cells. Nature 2004; 432: 396–401.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF . Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003; 100: 3983–3988.
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994; 367: 645–648.
Bonnet D, Dick JE . Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997; 3: 730–737.
Cox CV, Evely RS, Oakhill A, Pamphilon DH, Goulden NJ, Blair A . Characterization of acute lymphoblastic leukemia progenitor cells. Blood 2004; 104: 2919–2925.
Nijmeijer BA, Mollevanger P, van Zelderen-Bhola SL, Kluin-Nelemans HC, Willemze R, Falkenburg JH . Monitoring of engraftment and progression of acute lymphoblastic leukemia in individual NOD/SCID mice. Exp Hematol 2001; 29: 322–329.
Cobaleda C, Gutierrez-Cianca N, Perez-Losada J, Flores T, Garcia-Sanz R, Gonzalez M et al. A primitive hematopoietic cell is the target for the leukemic transformation in human philadelphia-positive acute lymphoblastic leukemia. Blood 2000; 95: 1007–1013.
Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 2005; 174: 6477–6489.
Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 2005; 106: 1565–1573.
Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 2007; 25: 1315–1321.
Hotfilder M, Rottgers S, Rosemann A, Schrauder A, Schrappe M, Pieters R et al. Leukemic stem cells in childhood high-risk ALL/t(9;22) and t(4;11) are present in primitive lymphoid-restricted CD34+CD19− cells. Cancer Res 2005; 65: 1442–1449.
Castor A, Nilsson L, Astrand-Grundstrom I, Buitenhuis M, Ramirez C, Anderson K et al. Distinct patterns of hematopoietic stem cell involvement in acute lymphoblastic leukemia. Nat Med 2005; 11: 630–637.
George AA, Franklin J, Kerkof K, Shah AJ, Price M, Tsark E et al. Detection of leukemic cells in the CD34(+)CD38(−) bone marrow progenitor population in children with acute lymphoblastic leukemia. Blood 2001; 97: 3925–3930.
Acknowledgements
This work was supported by grants from Ministry of Education, Culture, Sports, Science and Technology of Japan (FI) and National Institutes of Health (LDS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kong, Y., Yoshida, S., Saito, Y. et al. CD34+CD38+CD19+ as well as CD34+CD38−CD19+ cells are leukemia-initiating cells with self-renewal capacity in human B-precursor ALL. Leukemia 22, 1207–1213 (2008). https://doi.org/10.1038/leu.2008.83
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2008.83
Keywords
This article is cited by
-
Endothelial-derived small extracellular vesicles support B-cell acute lymphoblastic leukemia development
Cellular Oncology (2024)
-
Cancer stem cells: an insight into the development of metastatic tumors and therapy resistance
Stem Cell Reviews and Reports (2023)
-
Overcoming Wnt–β-catenin dependent anticancer therapy resistance in leukaemia stem cells
Nature Cell Biology (2020)
-
Ruxolitinib/nilotinib cotreatment inhibits leukemia-propagating cells in Philadelphia chromosome-positive ALL
Journal of Translational Medicine (2017)
-
Plastic CD34 and CD38 expression in adult B–cell precursor acute lymphoblastic leukemia explains ambiguity of leukemia-initiating stem cell populations
Leukemia (2017)