Abstract
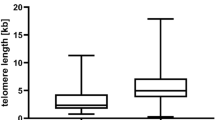
Myeloproliferative neoplasms (MPNs) are clonal stem cell disorders characterized by chronic proliferation of hematopoietic progenitors. We studied the telomere length (TL) of 335 MPN patients and 93 gender- and age-matched controls using a quantitative PCR method (relative TL calculated as the ratio of the amount of telomere DNA vs single-copy DNA: T/S ratio). TL was markedly reduced in MPN patients compared with controls (T/S 0.561 vs 0.990, P<0.001). In JAK2V617F MPN patients, TL correlated inversely with allelic burden (P<0.001). Patients homozygous for the mutation (allelic burden 90–100%) had the shortest TL, even when compared with patients with lower allele burdens consistent with a dominant heterozygous population (allelic burden 55–65%) (T/S 0.367 vs 0.497, P=0.037). This suggests that the high degree of proliferation of the MPN clone reduces TL and suggests the possibility that TL shortening may be indicative of progressive genomic instability during MPN progression. The TL of JAK2V617F-negative MPN patients was similar to JAK2V617F-positive counterparts (T/S 0.527 vs 0.507, P=0.603), suggesting that the yet-to-be-discovered causative mutation(s) impact the mutated stem cell similarly to JAK2V617F, and that TL measurement may prove useful in the diagnostic workup of JAK2V617F-negative MPN.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Greider CW . Telomere length regulation. Annu Rev Biochem 1996; 65: 337–365.
Hande MP, Samper E, Lansdorp P, Blasco MA . Telomere length dynamics and chromosomal instability in cells derived from telomerase null mice. J Cell Biol 1999; 144: 589–601.
Verfaillie CM, Pera MF, Lansdorp PM . Stem cells: hype and reality. Hematol Am Soc Hematol Educ Program 2002, 369–391.
Blasco MA . Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 2005; 6: 611–622.
von Zglinicki T, Martin-Ruiz CM . Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med 2005; 5: 197–203.
Drummond MW, Balabanov S, Holyoake TL, Brummendorf TH . Concise review: telomere biology in normal and leukemic hematopoietic stem cells. Stem Cells 2007; 25: 1853–1861.
Stewart SA, Weinberg RA . Telomeres: cancer to human aging. Annu Rev Cell Dev Biol 2006; 22: 531–557.
Greider CW . Telomerase activity, cell proliferation, and cancer. Proc Natl Acad Sci USA 1998; 95: 90–92.
Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL et al. Specific association of human telomerase activity with immortal cells and cancer. Science 1994; 266: 2011–2015.
Boultwood J, Fidler C, Shepherd P, Watkins F, Snowball J, Haynes S et al. Telomere length shortening is associated with disease evolution in chronic myelogenous leukemia. Am J Hematol 1999; 61: 5–9.
Boultwood J, Peniket A, Watkins F, Shepherd P, McGale P, Richards S et al. Telomere length shortening in chronic myelogenous leukemia is associated with reduced time to accelerated phase. Blood 2000; 96: 358–361.
Brummendorf TH, Holyoake TL, Rufer N, Barnett MJ, Schulzer M, Eaves CJ et al. Prognostic implications of differences in telomere length between normal and malignant cells from patients with chronic myeloid leukemia measured by flow cytometry. Blood 2000; 95: 1883–1890.
Engelhardt M, Mackenzie K, Drullinsky P, Silver RT, Moore MA . Telomerase activity and telomere length in acute and chronic leukemia, pre- and post-ex vivo culture. Cancer Res 2000; 60: 610–617.
Ohyashiki K, Ohyashiki JH, Iwama H, Hayashi S, Shay JW, Toyama K . Telomerase activity and cytogenetic changes in chronic myeloid leukemia with disease progression. Leukemia 1997; 11: 190–194.
Iwama H, Ohyashiki K, Ohyashiki JH, Hayashi S, Kawakubo K, Shay JW et al. The relationship between telomere length and therapy-associated cytogenetic responses in patients with chronic myeloid leukemia. Cancer 1997; 79: 1552–1560.
Brummendorf TH, Ersoz I, Hartmann U, Bartolovic K, Balabanov S, Wahl A et al. Telomere length in peripheral blood granulocytes reflects response to treatment with imatinib in patients with chronic myeloid leukemia. Blood 2003; 101: 375–376.
Ferraris AM, Pujic N, Mangerini R, Rapezzi D, Gallamini A, Racchi O et al. Clonal granulocytes in polycythaemia vera and essential thrombocythaemia have shortened telomeres. Br J Haematol 2005; 130: 391–393.
Ferraris AM, Mangerini R, Pujic N, Racchi O, Rapezzi D, Gallamini A et al. High telomerase activity in granulocytes from clonal polycythemia vera and essential thrombocythemia. Blood 2005; 105: 2138–2140.
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005; 7: 387–397.
Levine RL, Belisle C, Wadleigh M, Zahrieh D, Lee S, Chagnon P et al. X-inactivation-based clonality analysis and quantitative JAK2V617F assessment reveal a strong association between clonality and JAK2V617F in PV but not ET/MMM, and identifies a subset of JAK2V617F-negative ET and MMM patients with clonal hematopoiesis. Blood 2006; 107: 4139–4141.
Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW . Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet 1992; 51: 1229–1239.
Gale RE, Wheadon H, Linch DC . X-chromosome inactivation patterns using HPRT and PGK polymorphisms in haematologically normal and post-chemotherapy females. Br J Haematol 1991; 79: 193–197.
Vogelstein B, Fearon ER, Hamilton SR, Preisinger AC, Willard HF, Michelson AM et al. Clonal analysis using recombinant DNA probes from the X-chromosome. Cancer Res 1987; 47: 4806–4813.
Busque L, Mio R, Mattioli J, Brais E, Blais N, Lalonde Y et al. Nonrandom X-inactivation patterns in normal females: lyonization ratios vary with age. Blood 1996; 88: 59–65.
Allen RC, Nachtman RG, Rosenblatt HM, Belmont JW . Application of carrier testing to genetic counseling for X-linked agammaglobulinemia. Am J Hum Genet 1994; 54: 25–35.
Cawthon RM . Telomere measurement by quantitative PCR. Nucleic Acids Res 2002; 30: e47.
Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J et al. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension 2001; 37: 381–385.
Theocharides A, Boissinot M, Girodon F, Garand R, Teo SS, Lippert E et al. Leukemic blasts in transformed JAK2-V617F-positive myeloproliferative disorders are frequently negative for the JAK2-V617F mutation. Blood 2007; 110: 375–379.
Campbell PJ, Baxter EJ, Beer PA, Scott LM, Bench AJ, Huntly BJ et al. Mutation of JAK2 in the myeloproliferative disorders: timing, clonality studies, cytogenetic associations, and role in leukemic transformation. Blood 2006; 108: 3548–3555.
Lansdorp PM . Telomeres, stem cells, and hematology. Blood 2008; 111: 1759–1766.
Scheding S, Ersoz I, Hartmann U, Bartolvic K, Balabanov S, Salama A et al. Peripheral blood cell telomere length measurements indicate that hematopoietic stem cell turnover is not significantly increased in whole blood and apheresis PLT donors. Transfusion 2003; 43: 1089–1095.
Brummendorf TH, Rufer N, Baerlocher GM, Roosnek E, Lansdorp PM . Limited telomere shortening in hematopoietic stem cells after transplantation. Ann N Y Acad Sci 2001; 938: 1–7; discussion 7–8.
Ball SE, Gibson FM, Rizzo S, Tooze JA, Marsh JC, Gordon-Smith EC . Progressive telomere shortening in aplastic anemia. Blood 1998; 91: 3582–3592.
Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med 2005; 352: 1413–1424.
Muntoni A, Reddel RR . The first molecular details of ALT in human tumor cells. Hum Mol Genet 2005; 14 (Spec No. 2): R191–R196.
Dunham MA, Neumann AA, Fasching CL, Reddel RR . Telomere maintenance by recombination in human cells. Nat Genet 2000; 26: 447–450.
Acknowledgements
LM is an FRSQ scholar. DGG is an Investigator of the Howard Hughes Medical Institute, and a Doris Duke Charitable Foundation Distinguished Clinical Scientist Award recipient. RLL is an American Society of Hematology Basic Research Fellow Award recipient, a Doris Duke Charitable Foundation Clinical Scientist Development Award recipient and is the Geoffrey Beene Junior Chair at Memorial Sloan Kettering Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflicts of interest
No conflicts of interest are declared.
Rights and permissions
About this article
Cite this article
Bernard, L., Belisle, C., Mollica, L. et al. Telomere length is severely and similarly reduced in JAK2V617F-positive and -negative myeloproliferative neoplasms. Leukemia 23, 287–291 (2009). https://doi.org/10.1038/leu.2008.319
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2008.319
Keywords
This article is cited by
-
Targeted Therapy for MPNs: Going Beyond JAK Inhibitors
Current Hematologic Malignancy Reports (2023)
-
Genetic polymorphisms associated with telomere length and risk of developing myeloproliferative neoplasms
Blood Cancer Journal (2020)
-
Gender effect on phenotype and genotype in patients with post-polycythemia vera and post-essential thrombocythemia myelofibrosis: results from the MYSEC project
Blood Cancer Journal (2018)
-
Pharmacotherapy of Myelofibrosis
Drugs (2017)
-
Ruxolitinib therapy and telomere length in myelofibrosis
Blood Cancer Journal (2016)