Abstract
Objective:
To determine what barrier material used in hospital neonatal intensive care units most effectively blocks bacterial migration.
Study Design:
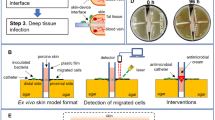
Bacterial migration distance was compared across simple and complex solid media using Escherichia coli, an early and common neonatal gut colonizer, and Staphylococcus aureus, a common skin bacterium, across polystyrene, medical-grade silicone, hydrocolloid dressing and transparent film dressing as barrier materials on complex solid media.
Results:
Bacterial migration was significantly greater on complex versus simple solid media. Bacteria migrated farthest beneath hydrocolloid dressing and transparent film dressing, while migration underneath polystyrene and medical-grade silicone was generally comparable to no barrier.
Conclusions:
Commonly used hydrocolloid dressing and transparent film dressing surprisingly increases bacterial migration, possibly by providing a wet capillary surface for bacteria to attach to or inducing biofilm formation. Using polystyrene or silicone to interface with the site of catheter insertion may best avoid a bacterial wicking phenomenon.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Harrison W, Goodman D . Epidemiologic trends in neonatal intensive care, 2007–2012. JAMA Pediatr 2015; 169 (9): 855–862.
Patrick SW, Kawai AT, Kleinman K, Jin R, Vaz L, Gay C et al. Health care-associated infections among critically ill children in the US, 2007–2012. Pediatrics 2014; 134 (4): 705–712.
Goldmann D . System failure versus personal accountability: the case for cleanhands. N Engl J Med 2006; 355 (2): 121–123.
Greenberg RG, Cochran KM, Smith PB, Edson BS, Schulman J, Lee HC et al. Effect of catheter dwell time on risk of central line-associated bloodstream infection in infants. Pediatrics 2015; 136 (6): 1080–1086.
Jean-Baptiste N, Benjabmin DK, Cohen-Wolkowiez M, Fowler VG, Laughon M, Clark RH et al. Coagulase-negative staphylococcal infections in the neonatal intensive care unit. Infect Control Hosp Epidemiol 2011; 32 (7): 679–686.
Harkes G, Dankert J, Feijen J . Bacterial migration along solid surfaces. Appl Environ Biol 1992; 58 (5): 1500–1505.
Frymier PD, Ford RM, Berg HC, Cummings PT . Three-dimensional tracking of motile bacteria near a solid planar surface. Proc Natl Acad Sci USA 1995; 92 (13): 6195–6199.
Darouiche RO, Safar H, Raad II . In vitro efficacy of antimicrobial-coated bladder catheters in inhibited bacterial migration along catheter surface. Can J Infect Dis 1997; 176 (4): 1109–1112.
Nickel JC, Costerton JW . Bacterial biofilms and catheters: a key to understanding bacterial strategies in catheter-associated urinary tract infection. Can J Infect Dis 1992; 3 (5): 261–267.
Reddy ST, Chung KK, McDaniel CJ, Darouiche RO, Landman J, Brennan AB . Micropatterned surfaces for reducing the risk of catheter-associated urinary tract infection: an in vitro study on the effect of Sharklet micropatterned surfaces to inhibit bacterial colonization and migration of uropathogenic Escherichia coli. J Endourol 2011; 25 (9): 1547–1552.
Tran K, Gibson A, Wong D, Tilahun D, Selock N, Good T et al. Designing a low-cost multifunctional infant incubator. J Lab Autom 2014; 19 (3): 332–337.
Dambkowski CL, Chehab EF, Shih JD, Venook R, Wall JK . In vitro assessment of bacterial colonisation rates of umbilical cord segments using three embodiments of a novel neonatal umbilical catheter protection device. BMJ Innov 2016; 2: 93–98.
Shoham Y, Kogan L, Weiss J, Tamir E, Krieger Y, Barnea Y et al. Wound ‘dechronification’ with negatively-charged polystyrene microspheres: a double-blind RCT. J Wound Care 2013; 22 (3): 144–146 148, 150–2.
Weissman O, Winkler E, Teot L, Remer E, Farber N, Bank J et al. Treatment of wounds following breast reduction and mastopexy with subsequent wound dehiscence with charged polystyrene microspheres. Wounds 2014; 26 (2): 37–42.
The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012; 486 (7402): 207–214.
Merenstein GB, Gardner SL . Handbook of Neonatal Intensive Care, 6th edn. Elsevier Health Sciences: Philadelphia, PA, 2006.
Tiffany KF, Burke BL, Collins-Odoms C, Oelberg DG . Current practice regarding the enteral feeding of high-risk newborns with umbilical catheters in situ. Pediatrics 2003; 112 (1 Pt.1): 20–23.
Soto SM, Bosch J, Jimenez de Anta MT, Villa J . Comparative study of virulence traits of escherichia coli clinical isolates causing early and late neonatal sepsis. J Clin Microbiol 2008; 46 (3): 1123–1125.
Wood TK, Gonzalez Barrios AF, Herzberg M, Lee J . Motility influences biofilm architecture in Escherichia coli. Appl Microbiol Biotechnol 2006; 72 (2): 361–367.
Surgical Materials Testing Laboratory Dressings Datacard: Bordered Granuflex. Available at http://www.dressings.org/Dressings/granufl-brd.html (accessed on 16 Dec 1997).
Garret TR, Bhakoo M, Zhang Z . Bacterial adhesion and biofilms on surfaces. Prog Nat Sci 2008; 18 (9): 1049–1056.
Sood A, Granick MS, Tomaselli NL . Wound dressings and comparative effectiveness data. Adv Wound Care 2014; 3 (8): 511–529.
Acknowledgements
We thank the Wallace H Coulter Foundation for financial support. We also thank the Stanford Department of Bioengineering for providing the facilities to conduct this study. This project was generously funded by the Stanford-Coulter Translational Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Shih, J., Wood, L., Dambkowski, C. et al. An in vitro bacterial surface migration assay underneath sterile barrier material commonly found in a hospital setting. J Perinatol 37, 848–852 (2017). https://doi.org/10.1038/jp.2017.28
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2017.28