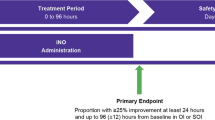
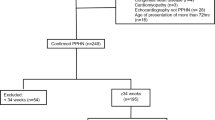
Abstract
The available evidence does not support the routine use of inhaled nitric oxide (iNO) in the care of premature infants. We present a case series of 22 preterm infants born after prolonged preterm premature rupture of membranes and oligohydramnios with respiratory failure. Oxygenation index decreased significantly after commencement of iNO.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Barrington KJ, Finer N . Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev 2010; (12): CD000509.
Askie LM, Ballard RA, Cutter GR, Dani C, Elbourne D, Field D et al. Inhaled nitric oxide in preterm infants: an individual patient data meta-analysis of randomized controlled trials. Pediatrics 2011; 128: 729–739.
Cole FS, Alleyne C, Barks JD, Boyle RJ, Carrol JL, Dokken D et al. NIH Consensus Development Conference statement: inhaled nitric-oxide therapy for premature infants. Pediatrics 2011; 127: 363–369.
Kilbride HW, Thibeault DW . Neonatal complications of preterm premature rupture of membranes. pathophysiology and management. Clin Perinatol 2001; 28: 761–785.
Chock VY, Van Meurs KP, Hintz SR, Ehrenkranz RA, Lemons JA, Kendrick DE et alNICHD Neonatal Research Network. Inhaled nitric oxide for preterm premature rupture of membranes, oligohydramnios, and pulmonary hypoplasia. Am J Perinatol 2009; 26: 317–322.
Geary C, Whitsett J . Inhaled nitric oxide for oligohydramnios induced pulmonary hypoplasia: a report of two cases and review of the literature. J Perinatol 2002; 22: 82–85.
Aikio O, Metsola J, Vuolteenaho R, Perhomaa M, Hallman M . Transient defect in nitric oxide generation after rupture of fetal membranes and responsiveness to inhaled nitric oxide in very preterm infants with hypoxic respiratory failure. J Pediatr 2012; 161: 397–403.
Kabra NS, Kluckow MR, Powell J . Nitric oxide in preterm infant with pulmonary hypoplasia. Indian J Pediatr 2004; 71: 427–429.
Peliowski A, Finer NN, Etches PC, Tierney AJ, Ryan CA . Inhaled nitric oxide for premature infants after prolonged rupture of membranes. J Pediatr 1995; 126: 450–453.
Williams O, Hutchings G, Debieve F, Debauche C . Contemporary neonatal outcome following rupture of membranes prior to 25 weeks with prolonged oligohydramnios. Early Hum Dev 2009; 85: 273–277.
Welzing L, Bagci S, Abramian A, Bartmann P, Berg C, Mueller A . CPAP combined with inhaled nitric oxide for treatment of lung hypoplasia and persistent foetal circulation due to prolonged PPROM. Early Hum Dev 2011; 87: 17–20.
Uga N, Ishii T, Kawase Y, Arai H, Tada H . Nitric oxide inhalation therapy in very low-birthweight infants with hypoplastic lung due to oligohydramnios. Pediatr Int 2004; 46: 10–14.
The International Neonatal Network. The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. Lancet 1993; 342: 193–198.
Parry G, Tucker J, Tarnow-Mordi W . UK Neonatal Staffing Study Collaborative Group. CRIB II: an update of the clinical risk index for babies score. Lancet 2003; 361: 1789–1791.
Van Meurs KP, Wright L, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK et al. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med 2005; 353: 13–22.
Acknowledgements
This work was supported by the EU FP7/2007–2013 under grant agreement no. 260777 (The HIP trial).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Semberova, J., O'Donnell, S., Franta, J. et al. Inhaled nitric oxide in preterm infants with prolonged preterm rupture of the membranes: a case series. J Perinatol 35, 304–306 (2015). https://doi.org/10.1038/jp.2015.2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2015.2