Abstract
Objective:
We used maternal immunoglobulin M (IgM), immunoglobulin G (IgG) avidity index (AI) and fetal ultrasonography (US) to effectively detect a congenital cytomegalovirus-infected fetus that would suffer neurological sequelae after birth.
Study Design:
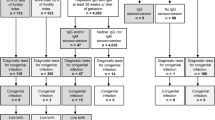
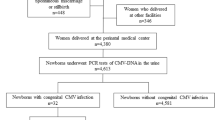
The detecting method was prospectively adapted to 1163 unselected pregnant women. IgM, IgG and IgG-AI were measured at the first prenatal examination (10.8±2.2 weeks of gestation). Advanced US was performed for the IgM-positive women at our center. The urine of 1163 neonates was examined via PCR. All infected neonates were followed for neurological development.
Result:
Most women (83.3%) were seropositive. Among them, 40 (4.1%) were IgM positive. Nine of forty (22.5%) had low AI, of which one showed abnormal US and suffered severe sequelae. The remaining eight had a normal US; however, one infant had hearing impairment. There were another three infected infants with normal development. Their mothers’ serological results were: IgM positive with high AI (n=1); IgG positive; IgM negative with high AI (n=1); and both IgG and IgM negative (n=1).
Conclusion:
This method enabled us to detect infected fetuses having severe sequelae. However, the problem remains of detecting infected fetuses that only have a hearing impairment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hyde TB, Schmid DS, Cannon MJ . Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol 2010; 20: 311–326.
Peckham CS . Cytomegalovirus infection: congenital and neonatal diseases. Scand J Infect Dis Suppl 1991; 80: 82–87.
Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA . The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med 1992; 326: 663–667.
Pass RF, Fowler KB, Boppana SB, Britt WJ, Stagno S . Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. J Clin Virol 2006; 35: 216–220.
Lazzarotto T, Guerra B, Gabrielli L, Lanari M, Landini MP . Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clin Microbiol Infect 2011; 17: 1285–1293.
Stagno S, Pass RF, Cloud G, Britt WJ, Henderson RE, Walton PD et al. Primary cytomegalovirus infection in pregnancy: incidence, transmission to fetus, and clinical outcome. JAMA 1986; 256: 1904–1984.
Adler SP, Finney JW, Manganello AM, Best AM . Prevention of child-to-mother transmission of cytomegalovirus among pregnant women. J Pediatr 2004; 145: 485–491.
Guera B, Simonazzi G, Puccetti C, Lanari M, Farina A, Lazzarotto T et al. Ultrasound prediction of symptomatic congenital cytomegalovirus infection. Am J Obstet Gynecol. 2008; 198 (4): 380 e1–e7.
Kenneson A, Cannon MJ . Review and meta-analysis of the epidemiology of congenital cytomegalovirus infection. Rev Med Virol 2007; 17: 253–276.
Revello MG, Gerna G . Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin. Microbiol. Rev 2002; 15: 680–715.
Lazzarotto T, Guera B, Lanari M, Gabrielli L, Landini MP . New advances in the diagnosis of congenital cytomegalovirus infection. J Clin Virol 2008; 41: 192–197.
Blackburn NK, Besselaar TG, Shoub BD, O’Connell KF . Differentiation of primary cytomegalovirus infection from reactivation using the urea denaturation test for measuring antibody avidity. J. Med. Virol 1991; 33: 6–9.
Lazzarotto T, Gabrielli L, Lanari M, Guerra B, Bellucci T, Sassi M et al. Congenital cytomegalovirus infection: Recent advances in the diagnosis of maternal infection. Hum Immunol 2004; 65: 410–415.
Munro SC, Hall B, Whybin LR, Leader L, Robertson P, Maine GT et al. Diagnosis of and screening for cytomegalovirus infection in pregnant women. J Clin. Microbiol 2005; 43 (9): 4713–4718.
Picone O, Vauloup-Fellous C, Cordier A-G, Châtelet IPD, Senat M-V, Frydman R et al. A 2-year study on cytomegalovirus infection during pregnancy in a French hospital. BJOG 2009; 116: 818–823.
Leruez-Ville M, Sellier Y, Salomon LJ, Stirnemann JJ, Jacquemard F, Ville Y . Prediction of fetal infection in cases with cytomegalovirus immunoglobulin M in the first trimester of pregnancy: a retrospective cohort. Clin Infect Dis 2013; 56: 1428–1435.
Benoist G, Salomon LJ, Mohlo M, Suarez B, Jacquemard F, Ville Y . The prognostic value of ultrasound abnormalities and biological parameters in blood of fetuses infected with cytomegalovirus. BJOG 2008; 115: 823–829.
Enders G, Bäder U, Lindemann L, Schalasta G, Daiminger A . Prenatal diagnosis of congenital cytomegalovirus infection in 189 pregnancies with known outcome. Prenatal Diagn 2001; 21: 362–377.
Binder ND, Buckmaster JW, Benda GI . Outcome for fetus with ascites and cytomegalovirus infection. Pediatrics 1988; 82: 100–103.
Crino JP . Ultrasound and fetal diagnosis of perinatal infection. Clin Obstet Gynecol 1999; 42: 71–80.
Lynch L, Daffos F, Emanuel D, Giovangrandi Y, Meisel R, Forestier F et al. Prenatal diagnosis of fetal cytomegalovirus infection. Am J Obstet Gynecol 1991; 165: 714–718.
Dollard SC, Grosse SD, Ross DS . New estimate of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007; 17: 355–363.
Numazaki K, Fujikawa T . Chronological changes of incidence and prognosis of children with asymptomatic congenital cytomegalovirus infection in Sapporo, Japan. BMC Infect Dis 2004; 4: 22.
Tagawa M, Minematus T, Masuzaki H, Ishimaru T, Moriuchi H . Seroepidemiological survey cytomegalovirus infection among pregnant women in Nagasaki, Japan. Pediatr Int 2010; 52: 459–462.
Vaulup-Fellous C, Picone O, Cordier AG, Parent-du-Chatelet I, Senat MV, Frydman R et al. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. J Clin Virol 2009; 46S: S49–S53.
Acknowledgements
We thank Drs Shigeki Tanaka, Syunichi Noda, Hiroyuki Watanabe and Takashi Matsumura for collecting sera and providing the patient data. This work was partly funded by a grant (No.19591899) from the Japan Ministry of Education, Culture, Sports, Science, and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kaneko, M., Sameshima, H., Minematsu, T. et al. Maternal IgG avidity, IgM and ultrasound abnormalities: combined method to detect congenital cytomegalovirus infection with sequelae. J Perinatol 33, 831–835 (2013). https://doi.org/10.1038/jp.2013.87
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2013.87
Keywords
This article is cited by
-
Primary cytomegalovirus infection during pregnancy and congenital infection: a population-based, mother–child, prospective cohort study
Journal of Perinatology (2021)
-
Anti-cytomegalovirus immunoglobulin M titer for congenital infection in first-trimester pregnancy with primary infection: a multicenter prospective cohort study
Journal of Perinatology (2017)