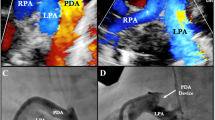
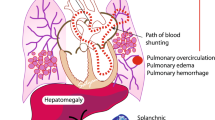
Abstract
Objective:
It remains unclear whether indomethacin (INDO) and/or surgical ligation (LIGATE) are necessary to improve outcomes in premature infants with a patent ductus arteriosus (PDA). We have adopted a conservative approach to PDA management that emphasizes waiting for spontaneous closure unless certain cardiorespiratory distress criteria are met.
Study Design:
This was a before-after observational study in infants born 501 to 1500 g in two distinct epochs. Era 1 (January 2005 to December 2007) featured traditional management with INDO and LIGATE used early to close all moderate and large PDAs in infants receiving any respiratory support. Era 2 (January 2008 to June 2009) emphasized modest fluid restriction, watchful waiting and limited INDO and LIGATE to only those infants with large PDAs who met certain cardiorespiratory distress criteria.
Result:
Era 1 included 139 infants with a PDA, mean (s.d.) gestational age 27.5 (2) weeks; Era 2 72 infants, mean (s.d.) gestational age 27.5 (2) weeks. In Era 2, INDO use significantly decreased (79% of infants to 26%, P<0.001), and 28 day total fluids decreased (140 vs. 130 ml kg−1 day−1, P<0.001). LIGATE rate was 45% in Era 1, 33% in Era 2 (P=0.11). There were no significant differences in supplemental oxygen, nasal continuous positive airway pressure, or mechanical ventilation days. There were no significant differences in mortality or individual morbidities. The combined outcome of chronic lung disease (CLD) or mortality after Day 7 significantly increased (Era 1, 40%, Era 2, 54%, P=0.04). More infants were discharged home with a PDA in Era 2, but most resolved spontaneously and the need for closure therapy after discharge from the neonatal intensive care unit (NICU) did not increase. Multiple regression analysis demonstrated Era 2 management did not predict an increased risk of one or more interlinked morbidities.
Conclusion:
Tolerance of the PDA with watchful waiting for spontaneous closure, modest fluid reduction, and less INDO use is a reasonable treatment strategy that is not associated with significant changes in NICU mortality or individual morbidities. We did note an increase in the combined outcome of CLD or mortality after Day 7, thus our investigation supports the urgency of a randomized controlled trial comparing traditional PDA management with a true control group similar to our Era 2 management to answer important questions of short and long-term outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Vermont Oxford Network. Vermont Oxford Network Annual Report. Section 2, Table 2.5. Vermont Oxford Network: Burlington, VT, 2009.
Bose CL, Laughon MM . Patent ductus arteriosus: lack of evidence for common treatments. Arch Dis Child Fetal Neonatal Ed 2007; 92: F498–F502.
Benitz WE . Treatment of persistent patent ductus arteriosus in preterm infants: time to accept the null hypothesis? J Perinatol 2010; 30: 241–252.
Hamrick SEG, Hansmann G . Patent ductus arteriosus of the preterm infant. Pediatrics 2010; 125: 1020–1030.
Vanhaesebrouck S, Zonnenberg I, Vandervoort P, Bruneel E, Van Hoestenberghe MR, Theyskens C . Conservative treatment for patent ductus arteriosus in the preterm. Arch Dis Child Fetal Neonatal Ed 2007; 92: F244–F247.
Pietz J, Achanti B, Lilien L, Stepka EC, Ken Mehta S . Prevention of necrotizing enterocolitis in preterm infants: a 20-year experience. Pediatrics 2007; 119: e164–e170.
Herrman K, Bose C, Laughon M . Spontaneous closure of the patent ductus arteriosus in very low birth weight infants following discharge from the neonatal unit. Arch Dis Child Fetal Neonatal Ed 2009; 94: F48–F50.
Vermont Oxford Network. Vermont Oxford Network Manual of Operations. Release 14.0. Vermont Oxford Network: Burlington, VT, 2010.
Kaempf JW, Campbell B, Sklar RS, Arduza C, Gallegos R, Zabari M et al. Implementing potentially better practices to improve neonatal outcomes after reducing postnatal dexamethasone use in premature infants born between 501 and 1250 grams. Pediatrics 2003; 111: e534–e541.
Payne NR, Finkelstein MJ, Liu M, Kaempf JW, Sharek PJ, Olsen S . NICU practices and outcomes associated with 9 years of quality improvement collaboratives. Pediatrics 2010; 125: 437–446.
Black N . Why we need observational studies to evaluate the effectiveness of health care. BMJ 1996; 312: 1215–1218.
Kabra NS, Schmidt BS, Roberts RS, Doyle LW, Papile L, Fanaroff A . Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: results from the trial of indomethacin prophylaxis in preterms. J Pediatrics 2007; 150: 229–234.
Madan JC, Kendrick D, Hagadorn JI, Frantz ID . Patent ductus arteriosus therapy: impact on neonatal and 18-month outcome. Pediatrics 2009; 123: 674–681.
Jhaveri N, Moon-Grady A, Clyman RI . Early surgical ligation versus a conservative approach for management of patent ductus arteriosus that fails to close after indomethacin treatment. J Pediatrics 2010; 157: 381–387.
Jones LJ, Craven PD, Attia J, Thakkinstain A, Wright I . Network meta-analysis of indomethacin versus ibuprofen versus placebo for PDA in preterm infants. Arch Dis Child Fetal Neonatal Ed 2011; 96: F45–F52.
Acknowledgements
We thank Catherine Wang and Nicole Tipping for their assistance with data collection. We also are grateful to the Vollum foundation and Northwest Newborn Specialists, PC for supporting this investigation. This investigation was supported by a grant from the Vollum Foundation and Northwest Newborn Specialists, PC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Kaempf, J., Wu, Y., Kaempf, A. et al. What happens when the patent ductus arteriosus is treated less aggressively in very low birth weight infants?. J Perinatol 32, 344–348 (2012). https://doi.org/10.1038/jp.2011.102
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2011.102
Keywords
This article is cited by
-
Outcomes in infants < 29 weeks of gestation following single-dose prophylactic indomethacin
Journal of Perinatology (2021)
-
Factors affecting the effectiveness of oral ibuprofen in the treatment of patent ductus arteriosus in preterm infants
International Journal of Clinical Pharmacy (2021)
-
Intravenous paracetamol in comparison with ibuprofen for the treatment of patent ductus arteriosus in preterm infants: a randomized controlled trial
European Journal of Pediatrics (2021)
-
Survey of PDA management in very low birth weight infants across Italy
Italian Journal of Pediatrics (2020)
-
Surgical Ligation Versus Percutaneous Closure of Patent Ductus Arteriosus in Very Low-Weight Preterm Infants: Which are the Real Benefits of the Percutaneous Approach?
Pediatric Cardiology (2018)