Abstract
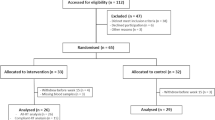
Regular exercise improves aging-induced deterioration of arterial stiffness, and is associated with elevated production of pentraxin 3 (PTX3) and anti-inflammatory as well as anti-atherosclerotic effects. However, the time-dependent effect of exercise training on arterial stiffness and PTX3 production remains unclear. The purpose of this study was to investigate the time course of the association between the effects of training on the circulating PTX3 level and arterial stiffness in middle-aged and older adults. Thirty-two healthy Japanese subjects (66.2±1.3 year) were randomly divided into two groups: training (exercise intervention) and sedentary controls. Subjects in the training group completed 8 weeks of aerobic exercise training (60–70% peak oxygen uptake (VO2peak) for 45 min, 3 days per week); during the training period, we evaluated plasma PTX3 concentration and carotid–femoral pulse wave velocity (cfPWV) every 2 wk. cfPWV gradually declined over the 8-week training period, and was significantly reduced after 6 and 8 week of exercise intervention (P<0.05). Plasma PTX3 level was significantly increased after 4 weeks of the intervention (P<0.05). In addition, the exercise training–induced reduction in cfPWV was negatively correlated with the percent change in plasma PTX3 level after 6 week (r=−0.54, P<0.05) and 8 weeks (r=−0.51, P<0.05) of the intervention, but not correlated at 4 weeks. Plasma PTX3 level was elevated at the early stage of the exercise training intervention, and was subsequently associated with training-induced alteration of arterial stiffness in middle-aged and older adults.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Safar ME, London GM . Therapeutic studies and arterial stiffness in hypertension: recommendations of the European Society of Hypertension. The Clinical Committee of Arterial Structure and Function. Working Group on Vascular Structure and Function of the European Society of Hypertension. J Hypertens 2000; 18: 1527–1535.
Arnett DK, Evans GW, Riley WA . Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol 1994; 140: 669–682.
Blacher J, Asmar R, Djane S, London GM, Safar ME . Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 1999; 33: 1111–1117.
Laurent S, Boutouyrie P, Asmar R, Laloux B, Guize L, Ducimetiere P et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37: 1236–1241.
Rowe JW . Clinical consequences of age-related impairments in vascular compliance. Am J Cardiol 1987; 60: 68G–71G.
Fujie S, Sato K, Miyamoto-Mikami E, Hasegawa N, Fujita S, Sanada K et al. Reduction of arterial stiffness by exercise training is associated with increasing plasma apelin level in middle-aged and older adults. PLoS ONE 2014; 9: e93545.
Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR . Aging, habitual exercise, and dynamic arterial compliance. Circulation 2000; 102: 1270–1275.
Birk GK, Dawson EA, Atkinson C, Haynes A, Cable NT, Thijssen DH et al. Brachial artery adaptation to lower limb exercise training: role of shear stress. J Appl Physiol 2012; 112: 1653–1658.
Tinken TM, Thijssen DH, Black MA, Cable NT, Green DJ . Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol 2008; 586: 5003–5012.
Green DJ, Maiorana A, O'Driscoll G, Taylor R . Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 2004; 561 (Pt 1): 1–25.
Schreuder TH, Green DJ, Nyakayiru J, Hopman MT, Thijssen DH . Time-course of vascular adaptations during 8 weeks of exercise training in subjects with type 2 diabetes and middle-aged controls. Eur J Appl Physiol 2015; 115: 187–196.
Jain S, Khera R, Corrales-Medina VF, Townsend RR, Chirinos JA . ‘Inflammation and arterial stiffness in humans’. Atherosclerosis 2014; 237: 381–390.
Kritchevsky SB, Cesari M, Pahor M . Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res 2005; 66: 265–275.
Amar J, Ruidavets JB, Peyrieux JC, Mallion JM, Ferrières J, Safar ME et al. C-reactive protein elevation predicts pulse pressure reduction in hypertensive subjects. Hypertension 2005; 46: 151–155.
Mahmud A, Feely J . Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension 2005; 46: 1118–1122.
Mäki-Petäjä KM, Hall FC, Booth AD, Wallace SM, Yasmin, Bearcroft PW et al. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation 2006; 114: 1185–1192.
Petersen AM, Pedersen BK . The anti-inflammatory effect of exercise. J Appl Physiol 2005; 98: 1154–1162.
Norata GD, Garlanda C, Catapano AL . The long pentraxin PTX3: a modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovasc Med 2010; 20: 35–40.
Norata GD, Marchesi P, Pulakazhi Venu VK, Pasqualini F, Anselmo A, Moalli F et al. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation 2009; 120: 699–708.
Dias AA, Goodman AR, Dos Santos JL, Gomes RN, Altmeyer A, Bozza PT et al. TSG-14 transgenic mice have improved survival to endotoxemia and to CLP-induced sepsis. J Leukoc Biol 2001; 69: 928–936.
Rolph MS, Zimmer S, Bottazzi B, Garlanda C, Mantovani A, Hansson GK . Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2002; 22: e10–e14.
Miyaki A, Maeda S, Choi Y, Akazawa N, Tanabe Y, Ajisaka R . Habitual aerobic exercise increases plasma pentraxin 3 levels in middle-aged and elderly women. Appl Physiol Nutr Metab 2012; 37: 907–911.
Miyaki A, Maeda S, Yoshizawa M, Misono M, Saito Y, Sasai H et al. Effect of weight reduction with dietary intervention on arterial distensibility and endothelial function in obese men. Angiology 2009; 60: 351–357.
Miyaki A, Maeda S, Otsuki T, Ajisaka R . Plasma pentraxin 3 concentration increases in endurance-trained men. Med Sci Sports Exerc 2011; 43: 12–17.
Suzuki S, Takeishi Y, Niizeki T, Koyama Y, Kitahara T, Sasaki T et al. Pentraxin 3, a new marker for vascular inflammation, predicts adverse clinical outcomes in patients with heart failure. Am Heart J 2008; 155: 75–81.
Yasunaga T, Ikeda S, Koga S, Nakata T, Yoshida T, Masuda N et al. Plasma pentraxin 3 is a more potent predictor of endothelial dysfunction than high-sensitive C-reactive protein. Int Heart J 2014; 55: 160–164.
Barnes JN, Trombold JR, Dhindsa M, Lin HF, Tanaka H . Arterial stiffening following eccentric exercise-induced muscle damage. J Appl Physiol 2010; 109: 1102–1108.
Huang CJ, Webb HE, Beasley KN, McAlpine DA, Tangsilsat SE, Acevedo EO . Cardiorespiratory fitness does not alter plasma pentraxin 3 and cortisol reactivity to acute psychological stress and exercise. Appl Physiol Nutr Metab 2014; 39: 375–380.
Slusher AL, Mock JT, Whitehurst M, Maharaj A, Huang CJ . The impact of obesity on pentraxin 3 and inflammatory milieu to acute aerobic exercise. Metabolism 2015; 64: 323–329.
Lan Q, Mercurius KO, Davies PF . Stimulation of transcription factors NF kappa B and AP1 in endothelial cells subjected to shear stress. Biochem Biophys Res Commun 1994; 201: 950–956.
Mohan S, Mohan N, Sprague EA . Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. Am J Physiol 1997; 273: C572–C578.
Altmeyer A, Klampfer L, Goodman AR, Vilcek J . Promoter structure and transcriptional activation of the murine TSG-14 gene encoding a tumor necrosis factor/interleukin-1-inducible pentraxin protein. J Biol Chem 1995; 270: 25584–25590.
Basile A, Sica A, d'Aniello E, Breviario F, Garrido G, Castellano M et al. Characterization of the promoter for the human long pentraxin PTX3. Role of NF-kappaB in tumor necrosis factor-alpha and interleukin-1beta regulation. J Biol Chem 1997; 272: 8172–8178.
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (KAKENHI: #26282199 and # 25560378 for M Iemitsu).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zempo-Miyaki, A., Fujie, S., Sato, K. et al. Elevated pentraxin 3 level at the early stage of exercise training is associated with reduction of arterial stiffness in middle-aged and older adults. J Hum Hypertens 30, 521–526 (2016). https://doi.org/10.1038/jhh.2015.105
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2015.105
This article is cited by
-
Impact of acute high-intensity interval exercise on plasma pentraxin 3 and endothelial function in obese individuals—a pilot study
European Journal of Applied Physiology (2021)
-
Aerobic fitness alters the capacity of mononuclear cells to produce pentraxin 3 following maximal exercise
European Journal of Applied Physiology (2018)