Abstract
Genetic variation in the human angiotensinogen gene (AGT) influences plasma AGT concentration and susceptibility to essential hypertension by a mechanism that remains to be clarified. When one or two additional copies of the gene were inserted by gene titration (by homologous recombination with gap-repair at the AGT locus), both plasma AGT and arterial pressure were elevated in the physiological range in the mouse. The causal dependency between plasma AGT and blood pressure and the relative contribution of the various tissues that express AGT to these two phenotypic parameters remained to be determined. To address these issues, we generated a transgenic mouse with overexpression of the mouse AGT gene restricted to the liver. The transgene was examined in two contrasted genetic backgrounds, the sodium-sensitive C57BL/6J and the sodium-resistant A/J. Transgenic and control male animals underwent continuous cardiovascular monitoring by telemetry for 14 days while under a standard sodium diet (0.2%). Moderate but significant increases in plasma AGT (40%, p = 0.01) and systolic blood pressure (4–6 mmHg, p ranging from 0.01 to <0.001) were observed in the sodium-sensitive background, but not in the sodium-resistant animals. Statistical analysis of a large number of consecutive, repeated measurements of blood pressure afforded power to detect small effects in the physiological range by use of advanced mixed models of analysis of variances and covariances. Although plasma renin activity was increased in the sodium-sensitive background, it did not reach statistical significance. These observations underline a potential contribution of systemic AGT to the mechanism of AGT-mediated hypertension, but the significance of sodium sensitivity in the genetic background suggests participation of the kidney in expression of the elevated blood pressure phenotype, a matter that will warrant further studies. They also highlight the challenge of identifying the contribution of individual genes in complex inheritance, as their effects are modulated by other genetic and environmental determinants.
Similar content being viewed by others
Introduction
The renin angiotensin system (RAS) participates in the regulation of blood pressure and vascular tone through both systemic and tissue sites (Paul et al. 2006). Angiotensinogen (AGT), the substrate from which angiotensin peptides are formed, is not only produced in the liver and delivered into the circulation but is also synthesized by various tissues including kidney, brain, heart, vascular wall, adipose tissue, and placenta (Danser 2003; Paul et al. 2006).
The hypothesis that genetic variation in the AGT gene may constitute an inherited predisposition to essential hypertension in humans (Lalouel and Rohrwasser 2007) was initially advanced on the basis of genetic linkage and allelic association in two independent samples of hypertensive siblings (Jeunemaitre et al. 1992). A common molecular variant in the gene, T235, was associated with both hypertension and increased plasma AGT (Jeunemaitre et al. 1992). Replication, albeit not achieved in all independent studies for reasons discussed elsewhere (Lalouel and Rohrwasser 2002), was corroborated in the aggregate (Kunz et al. 1997; Staessen et al. 1999).
These statistical observations provided no functional clue about the mechanism by which variation in AGT could affect blood pressure regulation. Subsequent experimental work showed that a common genetic variant in the proximal promoter of the gene, A/G(-6), in absolute linkage disequilibrium with the M/T(235) polymorphism, could affect the basal rate of transcription of the gene when assayed in vitro. The authors acknowledged, however, that “it is clearly not possible to directly extend the results of transfection experiments done with truncated AGT promoters in cultured cells to the function of an intact AGT gene at the level of the whole organism” (Inoue et al. 1997). Ready access to placental tissue afforded the test of an association between genetic polymorphism and AGT expression at the level of its transcript. Quantitative assays of the relative amounts of mRNA carrying each allele of the M/T 235 polymorphism in placental samples from heterozygous carriers provided in-vivo support for the differential expression hypothesis (Morgan et al. 1997).
Exploiting the species specificity of the renin–angiotensinogen reaction (Hatae et al. 1994; Takahashi et al. 1992; Yang et al. 1994), hetero-specific transgenic models overexpressing both human renin and human AGT led to marked hypertension and as such provided proof of principle that activation of the RAS leads to high blood pressure (Davisson et al. 1999; Lavoie and Sigmund 2003; Yang et al. 1994). The relative contribution of renal, systemic, and brain AGT in blood pressure regulation was thereafter investigated with the use of heterospecific overexpression of AGT and/or renin in the mouse. Animals overexpressing human AGT only exhibited normal blood pressure, confirming the species-specificity of the reaction (Takahashi et al. 1992; Yang et al. 1994). Animals overexpressing human AGT in the kidney or brain required the joint expression of human renin to bring about hypertension (Davisson et al. 1999; Yang et al. 1994), demonstrating the experimental power of the approach. By definition, these models probed the significance of AGT within a RAS with renin clamped at high levels.
It took the development of an original gene titration method by Smithies and colleagues, whereby a gene is duplicated within its natural genomic context (Smithies and Kim 1994), to demonstrate in intact mice that a moderate increase in the rate of expression of the homospecific AGT gene alone led to parallel increases in both plasma AGT and blood pressure (Kim et al. 1995; Smithies and Kim 1994). Remarkably, both parameters remained well within the normal physiological range and in close agreement with the variation in levels observed in humans.
Three questions remained to be addressed, however:
-
1
Is the rise in blood pressure mediated by the increase in plasma concentration of AGT, or are they simply correlated?
-
2
What are the relative contributions of systemic versus tissue expression of AGT to the observed increase in blood pressure?
-
3
What is the influence of the genetic background on the phenotypic expression of AGT?
In an attempt to address these questions, we have generated a transgenic mouse overexpressing mouse AGT in the liver. After confirmation that expression of the transgene was restricted to the liver, it was transferred by selective breeding into two inbred backgrounds, the sodium-sensitive C57BL/6J (B6) and the sodium-resistant A/J (AJ) strains. Our experiments show that whereas both blood pressure and plasma AGT exhibit moderate but sustained increases in the sodium-sensitive background, they remain unaltered in the sodium-resistant background. To achieve such inference required the statistical analysis of a large number of repeated observations using advanced mixed models of analysis of variances and covariances to test the significance of effects of the transgene on blood pressure in the physiological range, an issue of general relevance for the analysis of genetic effects of the magnitude expected in the common disease.
Materials and methods
Generation of the transgene
The cDNA of mouse AGT was introduced in the multiple cloning site of pBluescript II KS (Stratagene) between SalI and KpnI sites (Fig. 1a). The rabbit β-globin intronic and polyA flanked by KpnI restriction sites was generated by PCR and introduced 5′ of the AGT cDNA (5′ primer: 5′ GTG CTA GCG CGG CCT CGA ggt acc CGA TAT CTC TAG ACT GAG AAC TTC 3′, 3′ primer 5′ GTT GGC CGA TTC ATT AAT ggt acc GCA GTC GAG TTC ATA AGA GAA GAG 3′ with lowercase KpnI restriction sites) in the KpnI site. A polylinker containing NotI and XhoI restriction sites was subsequently inserted between SacI and SalI to accommodate the albumin enhancer-promoter 5′ of AGT cDNA (generously provided by Dr Palmiter; Pinkert et al. 1987). The entire construct was verified by DNA sequencing.
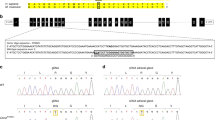
Generation of transgenic animals with liver-specific overexpression. a Schematic of the DNA construct used for microinjection and the relative location of the primers used to confirm the presence and specificity of expression of the transgene. b Identification of transgenic animals by PCR screen with sets of primers that amplify the two ends of the construct. c Detection of transgene expression pattern by RT-PCR with a set of primers that span the intronic sequence. d Transgene copy number in C57BL/6J and A/J
Microinjection and genotyping
The transgene construct (Fig. 1a) was linearized by Apa LI prior to microinjection by the transgenic core facility at University of Utah. Genomic DNA was extracted from the tails of offspring and screened for the presence of transgene by PCR using 5′ and 3′ amplicons: AEP2: 5′ GCT ACC TAT GCG ATC CAA ACA AC3′; T7: 5′TAA TAC GAC TCA CTA TAG GG3′ and Bgpas1: 5′CTG CTG TCC ATT CCT TAT TCC3′; T3: 5′AAT TAA CCC TCA CTA AAG GG3′. Amplifications were performed for 40 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s for both amplicons.
RNA isolation and expression analysis by RT-PCR
Total RNA was extracted from tissues using Trizol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was prepared from 1 μg RNA aliquots using SuperScript II reverse transcriptase (Invitrogen). From each cDNA preparation, the presence of transgene and GAPDH expression was monitored by PCR. The following primers were used: AGT: Tang1(5′): 5′ CTC TGC CCC AGT TGG AAA TC 3′ and AngTrans4(3′): CAG CCT GCA CCT GAG GAG TGA. The primers span the rabbit β-globin intron assuring specificity for the transgene cDNA and excluding amplification of genomic DNA. Amplification was performed for 30 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s. For glyceraldehydes-phosphate-dehydrogenase (GAPDH) amplification the following primers were used GAPDH-5′: 5′ TGG GAA GCT TGT CAT CAA CG3′ and GAPDH-3′: 5′ ATG CAG GGA TGA TGT TCT GG 3′. GAPDH amplification was performed for 25 cycles of 94°C for 30 s, 66°C for 30 s, and 72°C for 30 s.
Animal protocols
All experiments were performed under controlled conditions of temperature and lighting in age-matched F2 B6 and AJ male mice. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Utah (IACUC).
Experimental design and arterial pressure measurements by radiotelemetry
After two generations of crossing, seven male transgenic and eight control animals from each background, B6 and AJ, were implanted with telemetric devices (Data Sciences International, St Paul, MN, USA). Mice were anesthetized with isoflurane; the peritoneal cavity was opened and the transducer placed inside the abdomen. The probe was tunneled under the skin to the neck region. The neck region was dissected and the right common carotid artery identified. The probe was inserted into the artery and secured with sutures and Vetbond tissue adhesive. Subsequently, the abdominal wall and neck region were closed in layers using sutures and staples. The animals were allowed to recover for one week following surgery. Blood pressure (BP), heart rate, and activity were monitored for 10 s every 30 min, for 14 days continuously. Following blood pressure monitoring, mice were placed individually in metabolic cages, acclimatized for one week, and then studied for a three-day period. Daily food and water intake was monitored and 24-h water urine samples were collected. A 10-μl aliquot of the serine protease inhibitor 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF 0.5 M, Fisher, Pittsburgh, PA, USA) was added to the urine-collection tube to prevent protein degradation. At the end of the study period, animals were anesthetized and blood was collected by cardiac puncture before the animals were sacrificed. Plasma was extracted from the blood samples, frozen, and stored at −80°C.
Measurements of plasma AGT, PRA and aldosterone
AGT was measured by radioimmunoassay (RIA) as the release of AI after complete digestion by highly purified renin (Lantelme et al. 2002). PRA was measured by RIA as the released of AI after 30 min incubation of plasma samples at 37°C. Urinary aldosterone was determined using a commercial kit and following the manufacturer’s recommendations (Coat-A-Count, Diagnostic Products, Los Angeles, CA, USA) as described elsewhere (Lantelme et al. 2002).
Other measurements
Urinary sodium was measured by atomic absorption (Perkin–Elmer, Model 2380, Norwalk, CT, USA). Samples were diluted in deionized water (1:5,000). All dilutions were performed in duplicate; each sodium measurement consisted of the average of three consecutive readings. Urinary creatinine was measured using a commercial kit (Sigma) based on a modified Jaffé’s reaction. The samples were assayed following a 1:10 dilution. Each measurement was performed in duplicate, averaged and express in mg/dl.
Measurement of transgene copy number
Quantitative real-time PCR was performed to measure gene copy number in the two strains (A/J and B6). Genomic DNA was isolated from kidney tissue. AGT transgene and KAP gene copy numbers were measured by quantitative real-time PCR. Parallel amplification from plasmid DNA containing the exact amplicons ranging from 10−1 to 10−6 ng per reaction were used to derive absolute transcript amounts and to derive copy number ratios. The following primers were used for transgene amplification: 5′ GAACCAATGAAATGCGAGGT 3′ (located in albumin promoter) and 5′ GAAGGGGTGGATGTATACGC 3′ (located in AGT gene). The following primers were used for KAP amplification: 5′ GAGGTCTGGGCAGATGTTTC 3′ and 5′ CCACAGGACCTTCCTCAGAA 3′.
Statistical analysis
Individual values are presented as averages for plasma and urinary measurements (means ± standard error of the means, or SEM). Group means were compared by Student’s t test. Parameters measured by telemetry are presented either as 14-day averages at sampling times during the day (circadian pattern) or as day-time and night-time averages for each of the 14 days of monitoring. Day and night-time averages were defined on the basis of uninterrupted periods with minimal and marked activity, respectively.
Analysis of repeated measurements of BP (systolic (SBP), diastolic (DBP), and mean arterial pressure (MAP)) was carried out using a mixed model of analysis of variances and covariances (MM ANCOVA), using SPSS (SPSS, Chicago, IL, USA) and SAS software (Cary, NC, USA) (Jennrich and Schluchter 1986). This model was selected over standard general linear models (GLM) because:
-
1
it can handle random missing values without the artifact of interpolation;
-
2
it affords the definition of fixed effects not only for the transgene, but also for each day and time points at which telemetric data were sampled; and
-
3
it allows the investigator to model covariance structures that reflect the observed data as closely as feasible within the software/hardware limitations in the implementation of MM ANCOVA.
More specifically, a dependent variable y ijkl (such as SBP) in animal i, whose status is transgenic or control (fixed effect g j where j takes one of two modalities) measured at time k (with effect t k) and on day l (with effect d l) is expressed as:
where m is an overall mean and e ijkl is a random residual with a covariance structure defined so as to take into account the variances and covariances between repeated measurements sequentially ordered as a function of times and days of sampling. The objective is to test for the effect of the transgene while taking into account the large number of repeated observations in each animal to conserve statistical power. This power is largely lost when repeated measures are averaged without consideration of underlying significant sources of variation such as circadian rhythm. In addition to a possible effect of the transgene, the general model estimates and tests an effect of time of sampling, namely the circadian rhythm of BP, and an effect of day of observations to take into account the possibility of temporal trends in the course of the experiments.
To complete the definition of the mixed model of ANCOVA, the key challenge is to make proper assumptions regarding the structure of the variances and covariances of the residuals, e ijkl. Such assumptions are necessary, as a full model estimating each term of the n k × n l ANCOVA matrix would not only be saturated, leading to “overfitting”, but would also exceed the computational power of the software/hardware at hand. The task of the investigator is to recognize these features of the ANCOVA that need to be modeled in order to provide valid tests of the fixed effects.
This was achieved by balancing data-based observations against expediency imposed by computational limitations. In an independent experiment where we monitored BP at 30-min intervals over several months, generating a time-series with greater than 6,000 data points, we fitted an autoregressive integrated moving average (ARIMA) model affording the modeling of temporal trends and circadian variation (data not shown; SPSS Trends 10.0). This analysis showed that the structures of autocorrelations between successive observations (30-min sampling interval) exhibited rapid decay, closely approximating a second-order autoregressive pattern whereby measurement at time x depends significantly only on the two previous measurements, at time x − 1 and x − 2. These observations suggested that a first-order autoregressive model, where successive correlations at times 1, 2, …, n are estimated as ρ, ρ 2, …, ρ n, should provide an adequate description of the rapid decay of the autocorrelation structure. A second issue to be settled was the assumption which could be made about the variances at each successive time point, that is, whether such variances could be considered homogeneous, represented by a single parameter, σ 2, at all sampling points, or as a parameter varying with time of sampling. An examination of circadian rhythms revealed a manifest change in variances in the transitions from active to inactive states.
Consequently, within the confines of the computing limitations aforementioned, we performed analyses of the data under two nested autoregressive type-I models (AR1), with homogenous and heterogeneous variances, respectively. Where feasible through data reduction, two higher-order nested models were also considered—Toeplitz homogeneous and heterogeneous, or T and TH, respectively, where, in addition to the variance assumptions described above, we relaxed the covariance structure to accommodate different correlations between points separated by m intervals to be expressed as ρ m. Evidently, as the number of parameters increased rapidly, this extended model could be applied only to markedly reduced observations. All estimations were carried out by the method of restricted maximum likelihood, likelihood of nested models were compared, and statistical tests of fixed effects, both based on F and t tests, were evaluated for nested models.
Results
To investigate the effect of a modest but sustained increase in hepatic angiotensinogen production, we generated a transgenic animal overexpressing the mouse AGT gene in the liver. To test the potential significance of genetic background on phenotypic expression, we introduced the transgene into the sodium-sensitive (B6) and sodium-resistant (AJ) backgrounds. We placed animals in individual metabolic cages and we used radio-telemetry to perform continuous monitoring of blood pressure, heart rate, and activity in unrestrained conscious animals for 14 days. Metabolic, plasmatic, urinary, and physiological parameters were compared between transgenic and control animals.
Generation of transgenic mice
To achieve hepatic overexpression, we assembled the albumin enhancer and albumin promoter (Pinkert et al. 1987), the mouse AGT cDNA, and the rabbit β-globin intronic and polyA signal (Fig. 1a). Transgenic mice were developed by zygote microinjection at the Transgenic Core Facility of the University of Utah. The presence and the integrity of the transgene were assayed by PCR from tail DNA using two PCR amplicons spanning the 5′ and 3′ ends of the microinjected construct. Of three positive founders, two (53, 55) transmitted the transgene to progeny (Fig. 1b).
Liver-specific expression of the transgene
Tissue-specific expression of the transgene was tested by RT-PCR using RNA extracted from adipose tissue, heart, lung, kidney, brain, liver, small intestine, and muscle, in all lines generated. In one line positive for the transgene (53), expression was restricted to the liver (Fig. 1c); this line was chosen for further studies. In the other positive line (55), expression was not restricted to liver (data not shown) and the line was terminated. In line 53, we established that single-site integration occurred by fluorescence in-situ hybridization (data not shown).
Through three rounds of backcrossing, the transgene was transferred into predominantly sodium-sensitive B6 and sodium-resistant AJ backgrounds, as can be inferred from the recessive nature of the sodium sensitivity of B6 relative to AJ (Sugiyama et al. 2001; Woo and Kurtz 2003). Maintenance of transgene copy number of the transgene was investigated. We used quantitative PCR of genomic DNA to verify that stability of the transgene at the genomic level was maintained during breeding into both strains. This hypothesis is supported as the analysis shows that the estimated copy number of the transgene is similar after backcrossing into both strains (Fig. 1d).
Summary of metabolic parameters
Body weight and water intake did not vary significantly as a function of either transgene or strain (Table 1). Diuresis was higher in B6 animals, irrespective of genotype, but this difference did not reach statistical significance. Urinary sodium excretion, expressed as the ratio of sodium to creatinine, was lower in transgenic animals, but this difference was also not significant.
Plasma AGT, PRA, urinary aldosterone
In the B6 background, plasma AGT concentrations were increased by 40% in transgenic compared with control animals (transgenics = 13.15 ± 0.75 nM, n = 8; controls = 9.45 ± 1.02 nM, n = 6; p = 0.01; Fig. 2a). PRA was increased by 45% but this difference did not reach statistical significance (transgenics = 8.15 ± 0.77 ng AI/ml/h, n = 8; controls = 5.66 ± 1.06 ng AI/ml/h, n = 6; p = 0.07; Fig. 2b). Urinary aldosterone was not significantly different between the two groups when expressed relative to urinary creatinine (transgenics = 11.01 ± 1.41 ng/mg, n = 9, controls = 10.41 ± 0.60 ng/mg, n = 10, p = n.s.; Fig. 2c) or as 24-h absolute amount excreted (data not shown).
In contrast, in the AJ background plasma AGT (transgenics = 11.31 ± 1.08 nM, controls = 12.37 ± 0.89 nM), PRA (transgenics = 3.51 ± 1.20 ng AI/ml/h, n = 7 controls = 4.69 ± 0.73 ng AI/ml/h, n = 9), and urinary aldosterone (transgenics = 17.86 ± 2.00 ng/mg, n = 7, controls = 18.37 ± 1.91 ng/mg, n = 8) were not significantly different between transgenic and control animals (Fig. 2d, e, f).
Analysis of repeated measurements of blood pressure and related parameters
Continuous monitoring of blood pressure, heart rate, and activity were performed for 14 days at 30-min intervals. In B6 (seven transgenic and six control animals), graphical displays of blood pressure parameters, heart rate, and activity are provided in Figs. 3 and 4. In AJ (seven transgenic and eight control animals), only MAP measurements are given in Fig. 5. As expected, a marked circadian pattern was observed for all parameters, typical of the mouse in an animal resource environment, with no obvious differences in the pattern between transgenic animals and controls in either background (Figs. 3, 5a). Direct inspection of the data for either strain suggested greater variability of BP in active than inactive states or in transitions between these two states.
To test the effect of the transgene in each background, the data were reduced as a function of sampling rate in a number of ways in order to use the full power of the mixed model of analysis of variance and covariance as implemented in SPSS and SAS (see Materials and methods). The most representative results from such extensive analyses are presented in Table 2.
BP parameters were expressed as a function of fixed effects due to an overall mean, the presence/absence of the transgene, the time of sampling during the day (circadian effect), and the day of sampling (temporal trend). Variance and covariance structures were expressed following two models:
-
1
a Type I autoregressive model (AR1) and, when feasible;
-
2
a Toeplitz (T) model that relaxes some of the assumptions on the structures of covariances in the AR1 model.
In each instance, variances were considered either homogeneous (AR1, T) or heterogeneous (AR1H, TH).
Because the sampling rate of telemetric data has a marked effect on the number of parameters used to model variances and covariances of repeated measurements, T and TH models could be considered only when the data used in the analysis corresponded to sampling at 2-h intervals. All analyses involving jointly day and time as fixed effects revealed that day did not reach significance (data not shown). Consequently, all subsequent analyses were performed without this parameter in the model. Experimental results are thereafter presented to test transgene effects for either:
-
1
data averaged over days while accounting for circadian (time of sampling) variation; or
-
2
data averaged over time within periods of the day (day-time or night-time).
In the latter case, 30-min sampled data were averaged from 3 to 11 and 14 to 20, time segments defined for relatively stable degree of activity.
The most salient features of these analyses are summarized in Table 2. For any sampling rate, the circadian rhythm effect was very highly significant, with p values well below .001 in any model (data not shown). This is consistent with the fact that any BP parameter varied over a range of 20–30 mmHg throughout the day. Taking into account this major source of variation in BP data, tests of transgene effects reveals that:
-
1
the most critical element that needed to be included in the model was accounting for heterogeneity of the variances at sampling points during the day, as revealed by comparison of the corresponding likelihood ratios of the nested model AR1, AR1H and T, TH, respectively; and
-
2
refined modeling of covariance structures using Toeplitz models did not have a notable impact on the statistical inference of an effect of the transgene, as expected from our analysis of the rapid decay of autocorrelation with sampling intervals in our preliminary time-series analysis (data not shown).
Indeed, at 12-point sampling that afforded comparison of the likelihood of the AR1 to AR1H models or the T to TH models (Table 2a), the effect of the transgene on SBP (~6.9 mmHg) and MAP (~5.6 mmHg) reached high significance (p < .001) when heterogeneity of variances was taken into account. Not accounting for such variation can only artificially inflate the residual variance that appears in the denominator of the F-ratio statistics and blunt statistical power. Damping of the BP effects accountable to the transgene as more sampling points are considered while maintaining statistical significance when heterogeneity of variance is modeled (Table 2b, c) suggest that differences in circadian patterns may be at play, but this was beyond the reach of statistical testing under our model.
Daily variation in blood pressure
When BP parameters were averaged within days for active and inactive periods, similar conclusions were supported (Tables 2d, e), although power was somewhat reduced.
Blood pressure regressed for activity
To test whether differential activity between transgenic and control littermates could account for the differences observed in BP parameters, the analyses were repeated with activity included as a covariate. Similar conclusions were again supported (data not shown).
Analysis of the transgene in the AJ background
Only representative MAP data are presented in Fig. 5, as the transgene did not prove significant in all analyses performed (data not shown).
Discussion
The present experimental data in the mouse suggest that chronic, moderate overexpression of mouse AGT in the liver leads to increased plasma AGT and blood pressure, both in the physiological range, but that phenotypic expression may be dependent on genetic background. We will consider now the manner in which the data serve to address the three questions listed in the Introduction, the potential limitations of the study, and perspectives for the future.
On the relative contribution of systemic versus tissue expression of AGT in the observed blood pressure increase
Gene titration experiments (Kim et al. 2002; Smithies and Kim 1994) documented an increase in plasma AGT and BP in response to modest but sustained AGT expression in multiple natural tissues that express AGT. Our findings in this study were similar, even though mouse AGT overexpression was limited to the liver. This observation is restricted to the B6 background, as will be discussed later.
In B6 animals, plasma AGT was significantly raised by about 40%, quite in the range observed in humans as a function of genetic variation at the AGT locus (Jeunemaitre et al. 1992). In gene titration experiments, animals carrying three or four copies of the mouse AGT gene after several rounds of backcrossing into the B6 background exhibited 20 and 40% increases in plasma AGT, respectively (Smithies and Kim 1994), similar to our observations. Furthermore, in our study, day-to-day BP, whether systolic or mean, was significantly increased by 6–7 mmHg in transgenic animals compared with littermate controls over the entire range of the circadian rhythm and for both the active and the inactive periods, again rather similar to the gene titration experiments. From an analytical standpoint, we believe that the most significant lesson we learned was that repeated measurements can indeed provide substantial statistical power to infer gene effects in the physiological range, but that such inference required proper modeling of variance and covariance structures in the observations. Within the limitations imposed by current software and hardware, proper accounting of heterogeneity of variances was as critical as some modeling of the autocorrelation patterns to produce robust statistical inferences. That no significant effect of the transgene could be detected in the AJ background will be discussed in a subsequent section.
There was a trend toward greater PRA in transgenic B6 animals compared with littermate controls (p = .07). Despite the limited power of our study, this trend, in the face of a significant difference in plasma AGT concentrations, is consistent with the hypothesis that systemic overexpression of AGT is accompanied by a modest but sustained increase in circulating A-II formation. This is consistent with observations (Smithies and Kim 1994) and modeling studies (Kim et al. 2002) by Smithies and colleagues, which concluded that whereas increase plasma AGT is accompanied by a parallel decrease in circulating renin, as would be expected of the well-established negative feedback linking substrate and enzyme, this decrease does not restore steady-state values, thereby accounting for increased steady-state A-II formation. On the basis of metabolic studies performed in rats doubly transgenic for human renin and human AGT, the long catalytic cycle of the renin-angiotensinogen reaction in the circulation has been proposed as a mechanism to account for the decreased clearance of circulating renin induced by increased AGT concentrations (Bohlender et al. 2000). Further analysis of this mechanism, including the potential significance of stimulated AGT expression in the proximal nephron in this model, must await the generation of isogenic lines differing only by the presence/absence of the transgene in the homozygous state, which was in progress at the time we performed this work.
On the significance of the correlation between plasma AGT and BP
The parallel effects of the transgene on plasma AGT and BP in the B6 background suggest that the dependency between the two phenotypes may indeed be causal. This interpretation is supported by observations made in another transgenic model where rat AGT expression restricted to adipose tissue led to significant increases in both plasma AGT and blood pressure (Massiera et al. 2001). In two independent transgenic lines, these authors observed a 20–40% increase in plasma AGT, similar to that observed in our hepatic overexpression model. It is interesting to note that, while plasma AGT is generally assumed to originate in the liver, the adipose overexpression model suggests that adiposity can contribute to increases in both of these parameters, further supporting the hypothesis that elevation in plasma AGT can in itself contribute to increased blood pressure. While recent adenovirus-mediated ablation experiments strongly suggest that most, and perhaps all of circulating AGT originates in the liver under normal physiological states (Stec et al. 2002), there is also experimental evidence to support the hypothesis that, at least in the B6 inbred line, a high fat diet can increase AGT mRNA expression in intra-abdominal adipose tissue (Boustany et al. 2004, Rahmouni et al. 2004). The contribution of body mass index to plasma AGT independent of AGT genotype and ethnicity has been reported in a population-based, prospective study in humans (Bloem et al. 1995).
Although the magnitude of the increase in plasma AGT observed in the hepatic and adipose overexpression models were similar, the effect on blood pressure were much greater in the adipose model (~20 mmHg) than in our hepatic model (4–6 mmHg). Part of this apparent discrepancy may reflect an intrinsic difference in the method used for blood pressure measurements. Basal differences may be magnified by the stress associated with the restraint necessary to perform tail-cuff measurements (Gross and Luft 2003). This effect could result either from direct vasoconstriction or from a transient increase in A-II formation induced by acute renin release, amplifying differences in circulating plasma AGT, so long these remain below the K m of the reaction, as clearly would be the case in the adipose overexpression model. Unrestrained BP monitoring would more closely reflect differences in baseline blood pressure. Differences in genetic background could also in part account for the noted dissociation. Another factor that is likely to contribute to this noted difference is the known enhanced kinetics of mouse renin on rat substrate (Poulsen and Jacobsen 1986).
On the influence of genetic background on phenotypic expression of the transgene
The lack of any increase in plasma AGT in AJ animals, despite unambiguous evidence of the presence of the transgene in the corresponding animals, was an unexpected observation. A possible interpretation is that B6 and AJ differ with respect to other genes that contribute to sodium handling and RAS regulation. Similarly, significant differences in blood pressure were observed when the transgene was expressed in the B6 background but not in the AJ background. The possibility that the number of copies of the transgene, for which we have proved single site of insertion, was not maintained through crossing to bring the transgene in the AJ background can be ruled out, as we have verified that the copy number of the transgene, as measured by quantitative PCR, was similar in B6 and AJ animals (Fig. 1d). It is tempting to conclude that one or more of the strain-specific factors that may have affected phenotypic expression of the transgene on plasma AGT are also at play in arterial pressure regulation. Indeed, two studies document multiple QTLs contrasting the sodium sensitivity of B6 and AJ (Sugiyama et al. 2001, Woo and Kurtz 2003). Differential regulation of the RAS was also apparent when B6 animals when contrasted to the outbred CD1 line (Lantelme et al. 2002).
Although it is often ignored, genetic background may exert profound effects on the phenotypic expression of genetic differences, natural or generated experimentally, at the locus under investigation. This is particularly relevant for sodium sensitivity. Indeed, in a seminal review, Rapp (2000) subsumed his extensive experience with the Dahl Salt-sensitive and the Dahl-resistant strains by pointing out that “congenic strains can be constructed in two ways with a given pair of parental strains. The low-BP strain can be the donor and the high-BP strain the recipient, or vice versa. The main difference between the two is the genetic background. In the Dahl rats, for example, the genetic background of the R rats is not very permissive for expressing BP differences and so most congenic strains using Dahl rats have been made on the S genetic background”. The situation extends even to genetic manipulations of single mendelian inheritance. For example, mice harboring targeted disruption of the cystic fibrosis gene (Snouwaert et al. 1992) have a fibrotic lung phenotype in the C57BL/6J background that is not evident when the mutation is transferred into the BALB/c background (Haston et al. 2002).
In the absence of measurements of transcript levels of both endogenous and transgene AGT in liver, we cannot rule out the possibility that expression of the transgene in the A/J background may reflect a positional effect incurred during backcrossing. It is conceivable that we would have had the statistical power to resolve the expected modest difference in the level of these two transcripts, as the mechanism of translation should induce linear amplification in protein production. If so we would not have been able to differentiate between transcriptional and posttranscriptional effects in AGT expression. Plasma AGT as such encompasses but does not resolve the effects of all factors that may account for the level of the protein in plasma.
Limitations
This work must be considered preliminary in several respects. The underlying rationale for this initial investigation is that the cost, time, and effort required to collect data over the long-term must be validated and justified by a preliminary study that can only be of limited scope.
The experiments were conducted after three rounds of backcrossing in each of the B6 and AJ. Evidently, some genetic diversity that may affect the phenotypes under investigation may still segregate and induce greater variance than would be achieved in a quasi-homogeneous line, decreasing our statistical power. Regarding sodium sensitivity, however, it should be acknowledged that, in the two independent studies mentioned above (Sugiyama et al. 2001; Woo and Kurtz 2003), the analysis of blood pressure in segregating F1 and F2 between B6 and AJ clearly demonstrated that the major genetic determinants of sodium sensitivity, identified so far only as quantitative trait loci (QTL), behave as recessive traits in the B6 background. Consequently, limited rounds of backcrossing were sufficient to induce the expression of these recessive components of sodium sensitivity in most B6 animals and to negate their expression in all of the AJ animals investigated.
Breeding, genotyping and phenotyping, use of littermate controls, limitation to one gender, and the high cost of continuous monitoring of arterial pressure by telemetry altogether impose a stringent limitation on sample size. For continuous BP monitoring, our analysis was limited to eight transgenic and eight littermate controls, a rather typical sample size for studies of this nature. Measurements of plasma AGT and other assays were conducted in a more substantial number of animals, as less experimental constraints were in effect.
The mechanism by which increased systemic expression of AGT in the physiological range promotes increased BP remains to be clarified. Indeed, we cannot at this time resolve the moderate increase in BP observed in B6 animals from direct systemic effects of increased A-II formation from moderate stimulation of AGT expression in the proximal tubule. While the significance of chronic infusions of A-II at subpressor doses (3–5 ng/kg/min) suggests possible involvement of the kidney (Hall et al. 1999), future studies will be needed to test this hypothesis in our hepatic AGT overexpression model.
Perspective
Substantial data support the hypothesis that individual differences in AGT expression contribute to essential hypertension in humans. Animal have yielded strong proof of principle that a marked and global increase in AGT expression (Fukamizu et al. 1993; Kimura et al. 1992; Smithies and Kim 1994; Tokita et al. 1994; Yang et al. 1994) or in A-II formation (Cowley and McCaa 1976; Kobori et al. 2001) could indeed lead to marked hypertension. The Smithies titration model has proved that a similar effect could be observed in a physiological range that closely approximates the situation that may prevail in humans. The relevance of small perturbations in the physiological range, in contrast with marked disturbances in the pharmacological range, may unravel metabolic regulations that are particularly relevant to our understanding of a common disease in humans. Indeed, apportioning the contribution of systemic and tissue RAS in the physiological range should prove significant for drug targeting in the individual medicine of the future. The work we present here reveals that the technology is at hand to investigate small genetic effects in transgenic animals, provided adequate statistical analysis is applied to the data. It also emphasizes the challenge in demonstrating functional effects of genetic variants on complex phenotypes such as high blood pressure, as both the contribution of the genome and the lifetime experience of an individual modulates the expression of the gene under consideration.
References
Bloem LJ, Manatunga AK, Tewksbury DA, Pratt JH (1995) The serum angiotensinogen concentration and variants of the angiotensinogen gene in white and black children. J Clin Invest 95:948–953
Bohlender J, Menard J, Ganten D, Luft FC (2000) Angiotensinogen concentrations and renin clearance: implications for blood pressure regulation. Hypertension 35:780–786
Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA (2004) Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol 287:R943–R949
Cowley AW, McCaa RE (1976) Acute and chronic dose-response relationships for angiotensin, aldosterone, and arterial pressure at varying levels of sodium intake. Circ Res 39:788–797
Danser AH (2003) Local renin-angiotensin systems: the unanswered questions. Int J Biochem Cell Biol 35:759–768
Davisson RL, Ding Y, Stec DE, Catterall JF, Sigmund CD (1999) Novel mechanism of hypertension revealed by cell-specific targeting of human angiotensinogen in transgenic mice. Physiol Genomics 1:3–9
Fukamizu A, Sugimura K, Takimoto E, Sugiyama F, Seo MS, Takahashi S, Hatae T, Kajiwara N, Yagami K, Murakami K (1993) Chimeric renin-angiotensin system demonstrates sustained increase in blood pressure of transgenic mice carrying both human renin and human angiotensinogen genes. J Biol Chem 268:11617–11621
Gross V, Luft FC (2003) Exercising restraint in measuring blood pressure in conscious mice. Hypertension 41:879–881
Hall JE, Brands MW, Henegar JR (1999) Angiotensin II and long-term arterial pressure regulation: the overriding dominance of the kidney. J Am Soc Nephrol 10(Suppl 12):S258–S265
Haston CK, McKerlie C, Newbigging S, Corey M, Rozmahel R, Tsui LC (2002) Detection of modifier loci influencing the lung phenotype of cystic fibrosis knockout mice. Mamm Genome 13:605–613
Hatae T, Takimoto E, Murakami K, Fukamizu A (1994) Comparative studies on species-specific reactivity between renin and angiotensinogen. Mol Cell Biochem 131:43–47
Inoue I, Nakajima T, Williams CS, Quackenbush J, Puryear R, Powers M, Cheng T, Ludwig EH, Sharma AM, Hata A, Jeunemaitre X, Lalouel JM (1997) A nucleotide substitution in the promoter of human angiotensinogen is associated with essential hypertension and affects basal transcription in vitro. J Clin Invest 99:1786–1797
Jennrich RI, Schluchter MD (1986) Unbalanced repeated-measures models with structured covariance matrices. Biometrics 42:805–820
Jeunemaitre X, Soubrier F, Kotelevtsev YV, Lifton RP, Williams CS, Charru A, Hunt SC, Hopkins PN, Williams RR, Lalouel JM et al (1992) Molecular basis of human hypertension: role of angiotensinogen. Cell 71:169–180
Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O (1995) Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci USA 92:2735–2739
Kim HS, Lee G, John SW, Maeda N, Smithies O (2002) Molecular phenotyping for analyzing subtle genetic effects in mice: application to an angiotensinogen gene titration. Proc Natl Acad Sci USA 99:4602–4607
Kimura S, Mullins JJ, Bunnemann B, Metzger R, Hilgenfeldt U, Zimmermann F, Jacob H, Fuxe K, Ganten D, Kaling M (1992) High blood pressure in transgenic mice carrying the rat angiotensinogen gene. Embo J 11:821–827
Kobori H, Harrison-Bernard LM, Navar LG (2001) Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension 37:1329–1335
Kunz R, Kreutz R, Beige J, Distler A, Sharma AM (1997) Association between the angiotensinogen 235T-variant and essential hypertension in whites: a systematic review and methodological appraisal. Hypertension 30:1331–1337
Lalouel JM, Rohrwasser A (2002) Power and replication in case-control studies. Am J Hypertens 15:201–205
Lalouel JM, Rohrwasser A (2007) Genetic susceptibility to essential hypertension. Insight from angiotensinogen. Hypertension 49:597–603
Lantelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G, Thomas J, Xiao S, Ishigami T, Herrmann T, Terreros DA, Ward K, Lalouel JM (2002) Effects of dietary sodium and genetic background on angiotensinogen and Renin in mouse. Hypertension 39:1007–1014
Lavoie JL, Sigmund CD (2003) Minireview: overview of the renin-angiotensin system–an endocrine and paracrine system. Endocrinology 144:2179–2183
Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, Seydoux J, Meneton P, Teboul M (2001) Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. Faseb J 15:2727–2729
Morgan T, Craven C, Nelson L, Lalouel JM, Ward K (1997) Angiotensinogen T235 expression is elevated in decidual spiral arteries. J Clin Invest 100:1406–1415
Paul M, Poyan Mehr A, Kreutz R (2006) Physiology of local renin-angiotensin systems. Physiol Rev 86:747–803
Pinkert CA, Ornitz DM, Brinster RL, Palmiter RD (1987) An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice. Genes Dev 1:268–276
Poulsen K, Jacobsen J (1986) Is angiotensinogen a renin inhibitor and not the substrate for renin? J Hypertens 4:65–69
Rahmouni K, Mark AL, Haynes WG, Sigmund CD (2004) Adipose depot-specific modulation of angiotensinogen gene expression in diet-induced obesity. Am J Physiol Endocrinol Metab 286:E891–E895
Rapp JP (2000) Genetic analysis of inherited hypertension in the rat. Physiol Rev 80:135–172
Smithies O, Kim HS (1994) Targeted gene duplication and disruption for analyzing quantitative genetic traits in mice. Proc Natl Acad Sci USA 91:3612–3615
Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH (1992) An animal model for cystic fibrosis made by gene targeting. Science 257:1083–1088
Staessen JA, Kuznetsova T, Wang JG, Emelianov D, Vlietinck R, Fagard R (1999) M235T angiotensinogen gene polymorphism and cardiovascular renal risk. J Hypertens 17:9–17
Stec DE, Keen HL, Sigmund CD (2002) Lower blood pressure in floxed angiotensinogen mice after adenoviral delivery of Cre-recombinase. Hypertension 39:629–633
Sugiyama F, Churchill GA, Higgins DC, Johns C, Makaritsis KP, Gavras H, Paigen B (2001) Concordance of murine quantitative trait loci for salt-induced hypertension with rat and human loci. Genomics 71:70–77
Takahashi S, Fukamizu A, Hatae T, Yamada Y, Sugiyama F, Kajiwara N, Yagami K, Murakami K (1992) Species-specific kinetics of mouse renin contribute to maintenance of normal blood pressure in transgenic mice with overexpressed human angiotensinogen. J Vet Med Sci 54:1191–1193
Tokita Y, Franco-Saenz R, Reimann EM, Mulrow PJ (1994) Hypertension in the transgenic rat TGR(mRen-2)27 may be due to enhanced kinetics of the reaction between mouse renin and rat angiotensinogen [see comments]. Hypertension 23:422–427
Woo DD, Kurtz I (2003) Mapping blood pressure loci in (A/J x B6)F2 mice. Physiol Genomics 15:236–242
Yang G, Merrill DC, Thompson MW, Robillard JE, Sigmund CD (1994) Functional expression of the human angiotensinogen gene in transgenic mice. J Biol Chem 269:32497–32502
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gociman, B., Rohrwasser, A., Hillas, E. et al. Response to genetic manipulations of liver angiotensinogen in the physiological range. J Hum Genet 53, 775–788 (2008). https://doi.org/10.1007/s10038-008-0311-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-008-0311-1
Keywords
This article is cited by
-
Overexpression of mouse angiotensinogen in renal proximal tubule causes salt-sensitive hypertension in mice
American Journal of Hypertension (2012)