Abstract
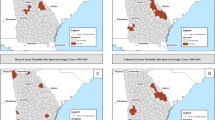
This study investigated the spatial distributions of mortality rates of six cancers: mesothelioma, lung cancer, intestinal cancer, nasopharyngeal and laryngeal cancer, liver cancer, and stomach cancer in Dayao using Geographic Information Systems. Relationships between the mortality rates of the six cancers and land use patterns were investigated by Pearson Correlation Coefficients. The results indicated that the mortality rates of nasopharyngeal and laryngeal cancer, lung cancer, intestinal cancer, and mesothelioma were significantly associated with outcropped asbestos. Both the proportions of farmland and urban area were positively related to the mortality rates of nasopharyngeal and laryngeal cancer, lung cancer, intestinal cancer, and mesothelioma, and significant negative correlations were found between the proportion of forestland and nasopharyngeal and laryngeal cancer and intestinal cancer. It can be concluded that naturally occurring asbestos may significantly elevate the mortality rates of nasopharyngeal and laryngeal cancer, intestinal cancer, lung cancer, and mesothelioma. Moreover, higher proportions of farmland, urban area, and lower proportions of forested land may elevate the mortality rate of the four cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kuntz S.W., Winters C.A., and Hill W.G., et al. Rural public health policy models to address an evolving environmental asbestos disaster. Public Health Nurs 2009: 26 (1): 70–78.
Wagner J.C., Sleggs C.A., and Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med 1960: 17: 260–271.
Gibbs G.W., and Berry G. Mesothelioma and asbestos. Regul Toxicol Pharmacol 2008: 52: S223–S231.
Dement J.M. Carcinogenicity of chrysotile asbestos: a case control study of textile workers. Cell Biol Toxicol 1991: 7: 59–65.
Constantopoulos S.H. Environmental mesothelioma associated with tremolite asbestos: lessons from the experiences of Turkey, Greece, Corsica, New Caledonia and Cyprus. Regul Toxicol Pharmacol 2008: 52: S110–S115.
Ribak J., and Ribak G. Human health effects associated with the commercial use of grunerite asbestos (amosite): Paterson, NJ; Tyler, TX; Uxbridge, UK. Regul Toxicol Pharmacol 2008: 52: S82–S90.
Edelman D.A. Laryngeal cancer and occupational exposure to asbestos. Int. Arch Occup Environ Health 1989: 61: 223–227.
Emri S., Demir A., and Dogan M., et al. Lung diseases due to environmental exposures to erionite and asbestos in Turkey. Toxicol Lett 2002: 127: 251–257.
Goodman M., Morgan R.W., and Ray R., et al. Cancer in asbestos-exposed occupational cohorts: a meta-analysis. Cancer Cause Control 1999: 10: 453–465.
Gamble J. Risk of gastrointestinal cancers from inhalation and ingestion of asbestos. Regul Toxicol Pharmacol 2008: 52: S124–S153.
Knoll L., Felten M.K., and Ackermann D., et al. Non-response bias in a surveillance program for asbestos-related lung cancer. J Occup Health 2011: 53: 16–22.
Treggiari M.M., and Weiss N.S. Occupational asbestos exposure and the incidence of non-Hodgkin lymphoma of the gastrointestinal tract: an ecologic study. Ann Epidemiol 2004: 14: 168–171.
Kanarek M.S. Mesothelioma from chrysotile asbestos: update. Ann Epidemiol 2011: 21: 688–697.
Neri M., Filiberti R., and Taioli E., et al. Pleural malignant mesothelioma, genetic susceptibility and asbestos exposure. Mutat Res-Fund Mol M 2005: 592: 36–44.
Driece H.A., Siesling S., Swuste P.H.J.J., and Burdorf A. Assessment of cancer risks due to environmental exposure to asbestos. J Expo Sci Environ Epidemiol 2010: 20: 478–485.
Pesch B., Taeger D., and Johnen G., et al. Cancer mortality in a surveillance cohort of German males formerly exposed to asbestos. Int J Hyg Environ Health 2010: 213 (1): 44–51.
IARC (International Agency for Research on Cancer.. IARC monographs. Supplement 7: Asbestos. International Agency for Research on Cancer, Lyon, 1987.
Hendrickx M. Naturally occurring asbestos in eastern Australia: a review of geological occurrence, disturbance and mesothelioma risk. Environ Geol 2009: 57: 909–926.
Senyiğit A., Babayiğit C., and Gökirmak M., et al. Incidence of malignant pleural mesothelioma due to environmental asbestos fiber exposure in the Southeast of Turkey. Respiration 2000: 67: 610–614.
Metintas S., Metintas M., and Ucgun I., et al. Malignant mesothelioma due to environmental exposure to asbestos. Chest 2002: 122: 2224–2229.
Luo S., Liu X., and Mu S., et al. Asbestos related diseases from environmental exposure to crocidolite in Da-yao, China. I. Review of exposure and epidemiological data. Occup Environ Med 2003: 60: 35–42.
Reid A., Heyworth J., and de Klerk N.H., et al. Cancer incidence among women and girls environmentally and occupationally exposed to blue asbestos at Wittenoom, Western Australia. Int J Cancer 2008: 122: 2337–2344.
Reid A., Heyworth J., and de Klerk N., et al. The mortality of women exposed environmentally and domestically to blue asbestos at Wittenoom, Western Australia. Occup Environ Med 2008: 65: 743–749.
Hasanoglu H.C., Yildirim Z., and Ermis H., et al. Lung cancer and mesothelioma in towns with environmental exposure to asbestos in Eastern Anatolia. Int Arch Occup Environ Health 2006: 79: 89–91.
Van Gosen B.S. The geology of asbestos in the United States and its practical applications. Environ Eng Geosci 2007: 13: 55–68.
Lee R.J., Strohmeier B.R., and Bunker K.L., et al. Naturally occurring asbestos—a recurring public policy challenge. J Hazard Mater 2008: 153: 1–21.
Favero-Longo S.E., Turci F., and Tomatis M., et al. The effect of weathering on ecopersistence, reactivity, and potential toxicity of naturally occurring asbestos and asbestiform minerals. J Toxicol Env Health A 2009: 72: 305–314.
Kurumatani N., and Kumagai S. Mapping the risk of mesothelioma due to neighborhood asbestos exposure. Am J Respir Crit Care Med 2008: 178: 624–629.
Rees D., Myers J.E., and Goodman K., et al. Case–control study of mesothelioma in South Africa. Am J Ind Med 1999: 35: 213–222.
Pira E., Pelucchi C., and Piolatto P.G., et al. First and subsequent asbestos exposures in relation to mesothelioma and lung cancer mortality. Brit J Cancer 2007: 97: 1300–1304.
Wei B., Yang L., and Zhang X., et al. Airborne crocidolite asbestos fibers in indoor and outdoor air in a rural area with naturally occurring asbestos from China. Aerosol Air Qual Res 2012 doi.10.4209/aaqr.2011.08.0121. (In press).
Peto J., Decarli A., and La Vecchia C., et al. The European mesothelioma epidemic. Brit J Cancer 1999: 79: 666–672.
Greillier L., and Astoul P. Mesothelioma and asbestos-related pleural diseases. Respiration 2008: 76: 1–15.
Lacourt P., Rolland P., and Gramond C., et al. Attributable risk in men in two French case-control studies on mesothelioma and asbestos. Eur J Epidemiol 2010: 25: 799–806.
Acknowledgements
The work described in this paper was financially supported by National Natural Science Foundation of China (project no. 41071064). We thankthe Center for Disease Control and Prevention of Dayao County, Yunnan. We also thank the Data Center for Resources and Environmental Sciences, Chinese Academy of Sciences (RESDC), for providing the data of land use patterns of Dayao.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wei, B., Jia, X., Ye, B. et al. Impacts of land use on spatial distribution of mortality rates of cancers caused by naturally occurring asbestos. J Expo Sci Environ Epidemiol 22, 516–521 (2012). https://doi.org/10.1038/jes.2012.63
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jes.2012.63