Abstract
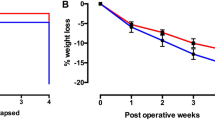
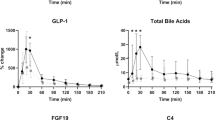
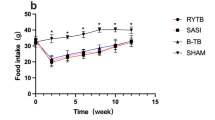
Roux-en-Y gastric bypass (RYGB) surgery is widely used in the management of morbid obesity. RYGB improves metabolism independently of weight loss by still unknown mechanisms. Bile acids (BAs) are good candidates to explain this benefit, since they regulate metabolic homeostasis and their systemic concentrations increase upon RYGB. Here we analyzed the mechanisms underlying the increase in systemic BA concentrations after RYGB and the role of the liver therein. To this aim, we used the Göttingen-like minipig, a human-size mammalian model, which allows continuous sampling and simultaneous analysis of pre-hepatic portal and systemic venous blood. BA concentrations and pool composition were measured in portal blood, containing intestinal reabsorbed BAs and compared to systemic blood during a standardized meal test before and after RYGB. Systemic total BA concentrations increased after RYGB, due to an increase in conjugated BAs. Interestingly, the ratio of portal:systemic conjugated BAs decreased after RYGB, indicating a role for the liver in systemic BA concentrations changes. In line, hepatic expression of BA transporter genes decreased after RYGB. Our results show that the increase in systemic BAs after surgery is due to decreased selective hepatic recapture. Thus, alterations in hepatic function contribute to the increase in systemic BAs after RYGB.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Eliasson B, Liakopoulos V, Franzén S, Näslund I, Svensson A-M, Ottosson J et al. Cardiovascular disease and mortality in patients with type 2 diabetes after bariatric surgery in Sweden: a nationwide, matched, observational cohort study. Lancet Diabetes Endocrinol 2015; 3: 847–854.
Tailleux A, Rouskas K, Pattou F, Staels B . Bariatric surgery, lipoprotein metabolism and cardiovascular risk. Curr Opin Lipidol 2015; 22: 317–324.
Caiazzo R, Lassailly G, Leteurtre E, Baud G, Verkindt H, Raverdy V et al. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5-year controlled longitudinal study. Ann Surg 2014; 260: 893–898; discussion 898–899.
Osto E, Doytcheva P, Corteville C, Bueter M, Dörig C, Stivala S et al. Rapid and body weight-independent improvement of endothelial and high-density lipoprotein function after Roux-en-Y gastric bypass: role of glucagon-like peptide-1. Circulation 2015; 131: 871–881.
Dirksen C, Bojsen-Møller KN, Jørgensen NB, Jacobsen SH, Kristiansen VB, Naver LS et al. Exaggerated release and preserved insulinotropic action of glucagon-like peptide-1 underlie insulin hypersecretion in glucose-tolerant individuals after Roux-en-Y gastric bypass. Diabetologia 2013; 56: 2679–2687.
Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007; 30: 1709–1716.
Sweeney TE, Morton JM . The human gut microbiome: a review of the effect of obesity and surgically induced weight loss. JAMA Surg 2013; 148: 563–569.
Osto M, Abegg K, Bueter M, le Roux CW, Cani PD, Lutz TA . Roux-en-Y gastric bypass surgery in rats alters gut microbiota profile along the intestine. Physiol Behav 2013; 119: 92–96.
Sweeney TE, Morton JM . Metabolic surgery: action via hormonal milieu changes, changes in bile acids or gut microbiota? A summary of the literature. Best Pract Res Clin Gastroenterol 2014; 28: 727–740.
Penney NC, Kinross JM, Newton RC, Purkayastha S . The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: A systematic review. Int J Obes 2005 2015; 17: 1565–1574.
Tremaroli V, Karlsson F, Werling M, Ståhlman M, Kovatcheva-Datchary P, Olbers T et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab 2015; 22: 228–238.
Bhutta HY, Rajpal N, White W, Freudenberg JM, Liu Y, Way J et al. Effect of Roux-en-Y gastric bypass surgery on bile acid metabolism in normal and obese diabetic rats. PloS One 2015; 10: e0122273.
Gerhard GS, Styer AM, Wood GC, Roesch SL, Petrick AT, Gabrielsen J et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care 2013; 36: 1859–1864.
Patti M-E, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obes Silver Spring Md 2009; 17: 1671–1677.
Simonen M, Dali-Youcef N, Kaminska D, Venesmaa S, Käkelä P, Pääkkönen M et al. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes Surg 2012; 22: 1473–1480.
Werling M, Vincent RP, Cross GF, Marschall H-U, Fändriks L, Lönroth H et al. Enhanced fasting and post-prandial plasma bile acid responses after Roux-en-Y gastric bypass surgery. Scand J Gastroenterol 2013; 48: 1257–1264.
Sachdev S, Wang Q, Billington C, Connett J, Ahmed L, Inabnet W et al. FGF 19 and bile acids increase following Roux-en-Y Gastric bypass but not after medical management in patients with type 2 diabetes. Obes Surg 2016; 26: 957–965.
Dutia R, Embrey M, O’Brien S, Haeusler RA, Agénor KK, Homel P et al. Temporal changes in bile acid levels and 12α-hydroxylation after Roux-en-Y gastric bypass surgery in type 2 diabetes. Int J Obes 2005 2015; 39: 806–813.
Steinert RE, Peterli R, Keller S, Meyer-Gerspach AC, Drewe J, Peters T et al. Bile acids and gut peptide secretion after bariatric surgery: A 1-year prospective randomized pilot trial. Obesity 2013; 21: E660–E668.
Ahmad NN, Pfalzer A, Kaplan LM . Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes 2013; 37: 1553–1559.
Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 2012; 153: 3613–3619.
Albaugh VL, Flynn CR, Cai S, Xiao Y, Tamboli RA, Abumrad NN . Early Increases in Bile Acids Post Roux-en-Y Gastric Bypass Are Driven by Insulin-Sensitizing, Secondary Bile Acids. J Clin Endocrinol Metab 2015; 100: E1225–E1233.
Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H . Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism 2009; 58: 1400–1407.
Jansen PLM, van Werven J, Aarts E, Berends F, Janssen I, Stoker J et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis Basel Switz 2011; 29: 48–51.
Mazuy C, Helleboid A, Staels B, Lefebvre P . Nuclear bile acid signaling through the farnesoid X receptor. Cell Mol Life Sci 2014; 72: 1631–1650.
Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B . Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 2009; 89: 147–191.
Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014; 509: 183–188.
Verhaeghe R, Zerrweck C, Hubert T, Tréchot B, Gmyr V, D’Herbomez M et al. Gastric bypass increases postprandial insulin and GLP-1 in nonobese minipigs. Eur Surg Res 2014; 52: 41–49.
Spinelli V, Lalloyer F, Baud G, Osto E, Kouach M, Daoudi M et al. Influence of Roux-en-Y gastric bypass on plasma bile acid profiles: a comparative study between rats, pigs and humans. Int J Obes 2016; 40: 1260–1267.
Broeders EPM, Nascimento EBM, Havekes B, Brans B, Roumans KHM, Tailleux A et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab 2015; 28: 418–426.
Ferrannini E, Camastra S, Astiarraga B, Nannipieri M, Castro-Perez J, Xie D et al. Increased bile acid synthesis and deconjugation after biliopancreatic diversion. Diabetes 2015; 26: 3377–3385.
Acknowledgements
We thank Audray Quenon, Amandine Descat, Bruno Derudas, Arnold Dive, Michel Pottier and Mathieu Fourdrinier for excellent technical assistance. VS was supported by a grant from the Fondation pour la Recherche Médicale (FRM) Grant FDT20140930804. This work was supported by Grants from Région Nord-Pas de Calais, FEDER, INSERM, ANR (FXREn), Société Francophone du Diabète (SFD), Institut Universitaire de France and European Genomic Institute for Diabetes (EGID, ANR-10-LABX-46), and European Commission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chávez-Talavera, O., Baud, G., Spinelli, V. et al. Roux-en-Y gastric bypass increases systemic but not portal bile acid concentrations by decreasing hepatic bile acid uptake in minipigs. Int J Obes 41, 664–668 (2017). https://doi.org/10.1038/ijo.2017.7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.7
This article is cited by
-
A pilot study about the development and characterization of a Roux en Y gastric bypass model in obese Yucatan minipigs
Scientific Reports (2021)
-
Roux-en-Y Gastric-Bypass and sleeve gastrectomy induces specific shifts of the gut microbiota without altering the metabolism of bile acids in the intestinal lumen
International Journal of Obesity (2019)
-
Bile acids in glucose metabolism and insulin signalling — mechanisms and research needs
Nature Reviews Endocrinology (2019)
-
Changes in Bile Acid Metabolism, Transport, and Signaling as Central Drivers for Metabolic Improvements After Bariatric Surgery
Current Obesity Reports (2019)