Abstract
Background/Objectives:
The phenomenon of adipocyte ‘beiging’ involves the conversion of non-classic brown adipocytes to brown-like adipose tissue with thermogenic, fat-burning properties, and this phenomenon has been shown in rodents to slow the progression of obesity-associated metabolic diseases. Rodent studies consistently report adipocyte beiging after endurance exercise training, indicating that increased thermogenic capacity in these adipocytes may underpin the improved health benefits of exercise training. The aim of this study was to determine whether prolonged endurance exercise training induces beige adipogenesis in subcutaneous adipose tissues of obese men.
Subjects/Methods:
Molecular markers of beiging were examined in adipocytes obtained from abdominal subcutaneous (AbSC) and gluteofemoral (GF) subcutaneous adipose tissues before and after 6 weeks of endurance exercise training in obese men (n=6, 37.3±2.3 years, 30.1±2.3 kg m–2).
Results:
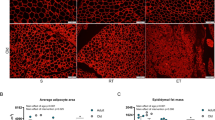
The mRNAs encoding the brown or beige adipocyte-selective proteins were very lowly expressed in AbSC and GF adipose tissues and exercise training did not alter the mRNA expression of UCP1, CD137, CITED, TBX1, LHX8 and TCF21. Using immunohistochemistry, neither multilocular adipocytes, nor UCP1 or CD137-positive adipocytes were detected in any sample. MicroRNAs known to regulate brown and/or beige adipose development were highly expressed in white adipocytes but endurance exercise training did not impact their expression.
Conclusions:
The present study reaffirms emerging data in humans demonstrating no evidence of white adipose tissue beiging in response to exercise training, and supports a growing body of work demonstrating divergence of brown/beige adipose location, molecular characterization and physiological function between rodents and humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Crowe S, Wu LE, Economou C, Turpin SM, Matzaris M, Hoehn KL et al. Pigment epithelium-derived factor contributes to insulin resistance in obesity. Cell Metab 2009; 10: 40–47.
Rosen ED, Spiegelman BM . What we talk about when we talk about fat. Cell 2014; 156: 20–44.
Tsiloulis T, Watt MJ . Exercise and the regulation of adipose tissue metabolism. Prog Mol Biol Transl Sci 2015; 135: 175–201.
Harms M, Seale P . Brown and beige fat: development, function and therapeutic potential. Nat Med 2013; 19: 1252–1263.
Carey AL, Formosa MF, Van Every B, Bertovic D, Eikelis N, Lambert GW et al. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia 2013; 56: 147–155.
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–1517.
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360: 1500–1508.
Mulya A, Kirwan JP . Brown and beige adipose tissue: therapy for obesity and its comorbidities? Endocrinol Metab Clin North Am 2016; 45: 605–621.
Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150: 366–376.
Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J . Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010; 285: 7153–7164.
Young P, Arch JR, Ashwell M . Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett 1984; 167: 10–14.
Bertholet AM, Kazak L, Chouchani ET, Bogaczynska MG, Paranjpe I, Wainwright GL et al. Mitochondrial patch clamp of beige adipocytes reveals UCP1-positive and UCP1-negative cells both exhibiting futile creatine cycling. Cell Metab 2017; 25: 811–822 e4.
Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J . UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep 2013; 5: 1196–1203.
Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X et al. A smooth muscle-like origin for beige adipocytes. Cell Metab 2014; 19: 810–820.
Rosenwald M, Wolfrum C . The origin and definition of brite versus white and classical brown adipocytes. Adipocyte 2014; 3: 4–9.
Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 2012; 7: e49452.
Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab 2013; 17: 798–805.
Carey AL, Vorlander C, Reddy-Luthmoodoo M, Natoli AK, Formosa MF, Bertovic DA et al. Reduced UCP-1 content in in vitro differentiated beige/brite adipocytes derived from preadipocytes of human subcutaneous white adipose tissues in obesity. PLoS ONE 2014; 9: e91997.
Stanford KI, Middelbeek RJ, Goodyear LJ . Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes 2015; 64: 2361–2368.
Knudsen JG, Murholm M, Carey AL, Bienso RS, Basse AL, Allen TL et al. Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PLoS One 2014; 9: e84910.
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481: 463–468.
Roberts LD, Bostrom P, O'Sullivan JF, Schinzel RT, Lewis GD, Dejam A et al. Beta-aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 2014; 19: 96–108.
Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014; 157: 1279–1291.
Kjaer M, Christensen NJ, Sonne B, Richter EA, Galbo H . Effect of exercise on epinephrine turnover in trained and untrained male subjects. J Appl Physiol (1985) 1985; 59: 1061–1067.
Sidossis LS, Porter C, Saraf MK, Borsheim E, Radhakrishnan RS, Chao T et al. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab 2015; 22: 219–227.
Nakhuda A, Josse AR, Gburcik V, Crossland H, Raymond F, Metairon S et al. Biomarkers of browning of white adipose tissue and their regulation during exercise- and diet-induced weight loss. Am J Clin Nutr 2016; 104: 557–565.
Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK et al. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J 2014; 281: 739–749.
Pino MF, Parsons SA, Smith SR, Sparks LM . Active individuals have high mitochondrial content and oxidative markers in their abdominal subcutaneous adipose tissue. Obesity (Silver Spring, MD) 2016; 24: 2467–2470.
Camera DM, Anderson MJ, Hawley JA, Carey AL . Short-term endurance training does not alter the oxidative capacity of human subcutaneous adipose tissue. Eur J Appl Physiol 2010; 109: 307–316.
Tsiloulis T, Pike J, Powell D, Rossello FJ, Canny BJ, Meex RC et al. Impact of endurance exercise training on adipocyte microRNA expression in overweight men. FASEB J 2017; 31: 161–171.
Lee P, Zhao JT, Swarbrick MM, Gracie G, Bova R, Greenfield JR et al. High prevalence of brown adipose tissue in adult humans. J Clin Endocrinol Metab 2011; 96: 2450–2455.
Friedlander MR, Mackowiak SD, Li N, Chen W, Rajewsky N . miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res 2012; 40: 37–52.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015; 43: e47.
Wickham H . ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag: New York, 2009.
Law CW, Chen Y, Shi W, Smyth GK . Voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol 2014; 15: R29.
Chen Y, Pan R, Pfeifer A . Regulation of brown and beige fat by microRNAs. Pharmacol Ther 2017; 170: 1–7.
Ringholm S, Grunnet Knudsen J, Leick L, Lundgaard A, Munk Nielsen M, Pilegaard H . PGC-1alpha is required for exercise- and exercise training-induced UCP1 up-regulation in mouse white adipose tissue. PLoS ONE 2013; 8: e64123.
Shen Y, Zhou H, Jin W, Lee HJ . Acute exercise regulates adipogenic gene expression in white adipose tissue. Biol Sport 2016; 33: 381–391.
Xu X, Ying Z, Cai M, Xu Z, Li Y, Jiang SY et al. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 2011; 300: R1115–R1125.
Rönn T, Volkov P, Tornberg Å, Elgzyri T, Hansson O, Eriksson KF et al. Extensive changes in the transcriptional profile of human adipose tissue including genes involved in oxidative phosphorylation after a 6-month exercise intervention. Acta Physiologica 2014; 211: 188–200.
Hilton C, Neville MJ, Karpe F . MicroRNAs in adipose tissue: their role in adipogenesis and obesity. Int J Obes (2005) 2013; 37: 325–332.
Arner P, Kulyte A . MicroRNA regulatory networks in human adipose tissue and obesity. Nat Rev Endocrinol 2015; 11: 276–288.
Price NL, Fernandez-Hernando C . miRNA regulation of white and brown adipose tissue differentiation and function. Biochim Biophys Acta 2016; 1861: 2104–2110.
Loh RKC, Kingwell BA, Carey AL . Human brown adipose tissue as a target for obesity management; beyond cold-induced thermogenesis. Obes Rev 2017; 18: 1227–1242.
Stanford KI, Goodyear LJ . Exercise regulation of adipose tissue. Adipocyte 2016; 5: 153–162.
Oldfield BJ, McLachlan EM . An analysis of the sympathetic preganglionic neurons projecting from the upper thoracic spinal roots of the cat. J Comp Neurol 1981; 196: 329–345.
Scheele C . Adipose adaptation to exercise training –increased metabolic rate but no signs of browning. Acta Physiologica 2014; 211: 11–12.
Kjaer M, Farrell PA, Christensen NJ, Galbo H . Increased epinephrine response and inaccurate glucoregulation in exercising athletes. J Appl Physiol (1985) 1986; 61: 1693–1700.
Helge JW, Stallknecht B, Pedersen BK, Galbo H, Kiens B, Richter EA . The effect of graded exercise on IL-6 release and glucose uptake in human skeletal muscle. J Physiol 2003; 546: 299–305.
Xue R, Lynes MD, Dreyfuss JM, Shamsi F, Schulz TJ, Zhang H et al. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat Med 2015; 21: 760–768.
Boquest AC, Shahdadfar A, Fronsdal K, Sigurjonsson O, Tunheim SH, Collas P et al. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell 2005; 16: 1131–1141.
Zuriaga MA, Fuster JJ, Gokce N, Walsh K . Humans and mice display opposing patterns of “browning” gene expression in visceral and subcutaneous white adipose tissue depots. Front Cardiovasc Med 2017; 4: 27.
Vosselman MJ, Hoeks J, Brans B, Pallubinsky H, Nascimento EB, van der Lans AA et al. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int J Obesity (2005) 2015; 39: 1696–1702.
Acknowledgements
We thank Professor Ken Ho (University of Queensland) for the brown adipose tissue sample. This work was supported by the National Health and Medical Research Council of Australia (NHMRC, APP1098972) and MJW is supported by a Senior Research Fellowship from the NHMRC (APP1077703).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tsiloulis, T., Carey, A., Bayliss, J. et al. No evidence of white adipocyte browning after endurance exercise training in obese men. Int J Obes 42, 721–727 (2018). https://doi.org/10.1038/ijo.2017.295
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.295
This article is cited by
-
Acute and long-term exercise adaptation of adipose tissue and skeletal muscle in humans: a matched transcriptomics approach after 8-week training-intervention
International Journal of Obesity (2023)
-
Effects of voluntary exercise on the expression of browning markers in visceral and subcutaneous fat tissue of normotensive and spontaneously hypertensive rats
Pflügers Archiv - European Journal of Physiology (2022)
-
Batokines: Mediators of Inter-Tissue Communication (a Mini-Review)
Current Obesity Reports (2022)
-
Exerkines in health, resilience and disease
Nature Reviews Endocrinology (2022)
-
Combined training increases thermogenic fat activity in patients with overweight and type 2 diabetes
International Journal of Obesity (2022)