Abstract
Background:
Few studies have examined both gene expression and DNA methylation profiles in subcutaneous adipose tissue (SAT) during long-term weight loss. Thus, molecular mechanisms in weight loss and regain remain elusive.
Participants/Methods:
We performed a 1-year weight loss intervention on 19 healthy obese participants (mean body mass index (BMI) 34.6 kg m−2) and studied longitudinal gene expression (Affymetrix Human Genome U133 Plus 2.0) and DNA methylation (Infinium HumanMethylation450 BeadChip) in SAT at 0, 5 and 12 months. To examine whether weight loss and acquired obesity produce reciprocal profiles, we verified our findings in 26 BMI-discordant monozygotic twin pairs.
Results:
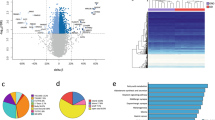
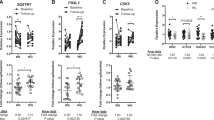
We found altered expression of 69 genes from 0 to 5’ months (short-term) weight loss. Sixty of these genes showed reversed expression in acquired obesity (twins). Altogether 21/69 genes showed significant expression–DNA methylation correlations. Pathway analyses revealed increased high-density lipoprotein-mediated lipid transport characteristic to short-term weight loss. After the fifth month, two groups of participants evolved: weight losers (WLs) and weight regainers (WRs). In WLs five genes were differentially expressed in 5 vs 12 months, three of which significantly correlated with methylation. Signaling by insulin receptor pathway showed increased expression. We further identified 35 genes with differential expression in WLs from 0 to 12 months (long-term) weight loss, with 20 showing opposite expression patterns in acquired obesity, and 16/35 genes with significant expression–DNA methylation correlations. Pathway analyses demonstrated changes in signal transduction, metabolism, immune system and cell cycle. Notably, seven genes (UCHL1, BAG3, TNMD, LEP, BHMT2, EPDR1 and OSTM1) were found to be downregulated during both short- and long-term weight loss.
Conclusions:
Our study indicates short- and long-term weight loss influences in transcription and DNA methylation in SAT of healthy participants. Moreover, we demonstrate that same genes react in an opposite manner in weight loss and acquired obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Coelho M, Oliveira T, Fernandes R . Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 2013; 9: 191–200.
Scherer PE . Adipose tissue—from lipid storage compartment to endocrine organ. Diabetes 2006; 55: 1537–1545.
Ahima RS, Flier JS . Adipose tissue as an endocrine organ. Trends Endocrin Met 2000; 11: 327–332.
Fruhbeck G, Gomez-Ambrosi J, Muruzabal FJ, Burrell MA . The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab 2001; 280: E827–E847.
Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly Shannon C et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 23: 591–601.
Heinonen S, Buzkova J, Muniandy M, Kaksonen R, Ollikainen M, Ismail K et al. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes 2015; 64: 3135–3145.
Naukkarinen J, Heinonen S, Hakkarainen A, Lundbom J, Vuolteenaho K, Saarinen L et al. Characterising metabolically healthy obesity in weight-discordant monozygotic twins. Diabetologia 2014; 57: 167–176.
Calabrò P, Golia E, Maddaloni V, Malvezzi M, Casillo B, Marotta C et al. Adipose tissue-mediated inflammation: the missing link between obesity and cardiovascular disease? Intern Emerg Med 2009; 4: 25–34.
Hajer GR, van Haeften TW, Visseren FLJ . Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 2008; 29: 2959–2971.
Lee J, Wu YY, Fried SK . Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr 2010; 13: 371–376.
Lemoine AY, Ledoux S, Larger E . Adipose tissue angiogenesis in obesity. Thromb Haemostasis 2013; 110: 661–668.
Pietilainen KH, Ismail K, Jarvinen E, Heinonen S, Tummers M, Bollepalli S et al. DNA methylation and gene expression patterns in adipose tissue differ significantly within young adult monozygotic BMI-discordant twin pairs. Int J Obes 2016; 40: 654–661.
Dahlman I, Linder K, Arvidsson Nordström E, Andersson I, Lidén J, Verdich C et al. Changes in adipose tissue gene expression with energy-restricted diets in obese women. Am J Clin Nutr 2005; 81: 1275–1285.
Johansson LE, Danielsson AP, Parikh H, Klintenberg M, Norström F, Groop L et al. Differential gene expression in adipose tissue from obese human subjects during weight loss and weight maintenance. Am J Clin Nutr 2012; 96: 196–207.
Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J 2004; 18: 1657–1669.
Mutch DM, Pers TH, Temanni MR, Pelloux V, Marquez-Quinones A, Holst C et al. A distinct adipose tissue gene expression response to caloric restriction predicts 6-mo weight maintenance in obese subjects. Am J Clin Nutr 2011; 94: 1399–1409.
Campbell KL, Foster-Schubert KE, Makar KW, Kratz M, Hagman D, Schur EA et al. Gene expression changes in adipose tissue with diet- and/or exercise-induced weight loss. Cancer Prev Res 2013; 6: 217–231.
Mardinoglu A, Heiker JT, Gärtner D, Björnson E, Schön MR, Flehmig G et al. Extensive weight loss reveals distinct gene expression changes in human subcutaneous and visceral adipose tissue. Sci Rep 2015; 5: 14841.
Bouchard L, Rabasa-Lhoret R, Faraj M, Lavoie M-È, Mill J, Pérusse L et al. Differential epigenomic and transcriptomic responses in subcutaneous adipose tissue between low and high responders to caloric restriction. Am J Clin Nutr 2010; 91: 309–320.
Rönn T, Volkov P, Davegårdh C, Dayeh T, Hall E, Olsson AH et al. A six months exercise intervention influences the genome-wide dna methylation pattern in human adipose tissue. PLoS Genet 2013; 9: e1003572.
Multhaup Michael L, Seldin Marcus M, Jaffe Andrew E, Lei X, Kirchner H, Mondal P et al. Mouse-human experimental epigenetic analysis unmasks dietary targets and genetic liability for diabetic phenotypes. Cell Metab 21: 138–149.
Benton MC, Johnstone A, Eccles D, Harmon B, Hayes MT, Lea RA et al. An analysis of DNA methylation in human adipose tissue reveals differential modification of obesity genes before and after gastric bypass and weight loss. Genome Biol 2015; 16: 8.
Pietiläinen KH, Kaye S, Karmi A, Suojanen L, Rissanen A, Virtanen KA . Agreement of bioelectrical impedance with dual-energy X-ray absorptiometry and MRI to estimate changes in body fat, skeletal muscle and visceral fat during a 12-month weight loss intervention. Br J Nutr 2013; 109: 1910–1916.
Rappou E, Jukarainen S, Rinnankoski-Tuikka R, Kaye S, Heinonen S, Hakkarainen A et al. Weight loss is associated with increased NAD+/SIRT1 expression but reduced PARP activity in white adipose tissue. J Clin Endocrinol Metab 2016; 101: 1263–1273.
Kaprio J . The Finnish Twin Cohort Study: an update. Twin Res Hum Genet 2013; 16: 157–162.
Baecke JAH, Burema J, Frijters JER A . Short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982; 36: 936–942.
Heinonen S, Saarinen L, Naukkarinen J, Rodriguez A, Fruhbeck G, Hakkarainen A et al. Adipocyte morphology and implications for metabolic derangements in acquired obesity. Int J Obes 2014; 38: 1423–1431.
Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP . Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 2003; 31: e15.
Dai M, Wang P, Boyd AD, Kostov G, Athey B, Jones EG et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res 2005; 33: e175.
Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D et al. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics 2013; 29: 189–196.
Johnson WE, Li C, Rabinovic A . Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007; 8: 118–127.
Naeem H, Wong NC, Chatterton Z, Hong MKH, Pedersen JS, Corcoran NM et al. Reducing the risk of false discovery enabling identification of biologically significant genome-wide methylation status using the HumanMethylation450 array. BMC Genomics 2014; 15: 51.
Smyth Gordon K . Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 2004; 3: 1–25.
Efron B, Tibshirani R . On testing the significance of sets of genes. Ann Appl Stat 2007; 1: 107–129.
Sun K, Kusminski CM, Scherer PE . Adipose tissue remodeling and obesity. J Clin Invest 2011; 121: 2094–2101.
Somekawa S, Imagawa K, Hayashi H, Sakabe M, Ioka T, Sato GE et al. Tmem100, an ALK1 receptor signaling-dependent gene essential for arterial endothelium differentiation and vascular morphogenesis. Proc Natl Acad Sci USA 2012; 109: 12064–12069.
Weng H-J, Patel Kush N, Jeske Nathaniel A, Bierbower Sonya M, Zou W, Tiwari V et al. Tmem100 is a regulator of TRPA1-TRPV1 complex and contributes to persistent pain. Neuron 2015; 85: 833–846.
Palming J, Sjöholm K, Jernås M, Lystig TC, Gummesson A, Romeo S et al. The expression of NAD(P)H:quinone oxidoreductase 1 is high in human adipose tissue, reduced by weight loss, and correlates with adiposity, insulin sensitivity, and markers of liver dysfunction. J Clin Endocrinol Metab 2007; 92: 2346–2352.
Rashid S, Genest J . Effect of obesity on high-density lipoprotein metabolism. Obesity 2007; 15: 2875–2888.
Strycharz J, Drzewoski J, Szemraj J, Sliwinska A . Is p53 involved in tissue-specific insulin resistance formation? Oxid Med Cell Longev 2017; 2017: 23.
Sakai T, Sakaue H, Nakamura T, Okada M, Matsuki Y, Watanabe E et al. Skp2 controls adipocyte proliferation during the development of obesity. J Biol Chem 2007; 282: 2038–2046.
Corvera S, Gealekman O . Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim Biophys Acta 2014; 1842: 463–472.
Onogi Y, Wada T, Kamiya C, Inata K, Matsuzawa T, Inaba Y et al. PDGFRβ regulates adipose tissue expansion and glucose metabolism via vascular remodeling in diet-induced obesity. Diabetes 2017; 66: 1008–1021.
Zamani N, Brown CW . Emerging roles for the transforming growth factor-β superfamily in regulating adiposity and energy expenditure. Endocr Rev 2011; 32: 387–403.
Böttcher Y, Unbehauen H, Klöting N, Ruschke K, Körner A, Schleinitz D et al. Adipose tissue expression and genetic variants of the bone morphogenetic protein receptor 1A gene (BMPR1A) are associated with human obesity. Diabetes 2009; 58: 2119–2128.
Yang HJ, Xia YY, Wang L, Liu R, Goh KJ, Ju PJ et al. A novel role for neural cell adhesion molecule in modulating insulin signaling and adipocyte differentiation of mouse mesenchymal stem cells. J Cell Sci 2011; 124: 2552–2560.
Lodhi Irfan J, Semenkovich Clay F . Peroxisomes: a nexus for lipid metabolism and cellular signaling. Cell Metab 2014; 19: 380–392.
Gandhi PU, Gaggin HK, Belcher AM, Harisiades JE, Basile A, Falco A et al. Analysis of BAG3 plasma concentrations in patients with acutely decompensated heart failure. Clin Chim Acta 2015; 445: 73–78.
Rosati A, Graziano V, De Laurenzi V, Pascale M, Turco MC . BAG3: a multifaceted protein that regulates major cell pathways. Cell Death Dis 2011; 2: e141.
Rizkalla SW, Prifti E, Cotillard A, Pelloux V, Rouault C, Allouche R et al. Differential effects of macronutrient content in 2 energy-restricted diets on cardiovascular risk factors and adipose tissue cell size in moderately obese individuals: a randomized controlled trial. Am J Clin Nutr 2012; 95: 49–63.
Senol-Cosar O, Flach RJR, DiStefano M, Chawla A, Nicoloro S, Straubhaar J et al. Tenomodulin promotes human adipocyte differentiation and beneficial visceral adipose tissue expansion. Nat Commun 2016; 7: 10686.
Arvidsson E, Viguerie N, Andersson I, Verdich C, Langin D, Arner P . Effects of different hypocaloric diets on protein secretion from adipose tissue of obese women. Diabetes 2004; 53: 1966–1971.
Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab 2000; 85: 3338–3342.
Aguilera CM, Gomez-Llorente C, Tofe I, Gil-Campos M, Cañete R, Gil Á . Genome-wide expression in visceral adipose tissue from obese prepubertal children. Int J Mol Sci 2015; 16: 7723–7737.
Ye F, Zhang H, Yang Y-X, Hu H-D, Sze SK, Meng W et al. Comparative proteome analysis of 3T3-L1 adipocyte differentiation using iTRAQ-coupled 2D LC-MS/MS. J Cell Biochem 2011; 112: 3002–3014.
Liu Y, Zhang Z-c, Qian S-w, Zhang Y-y, Huang H-y, Tang Y et al. MicroRNA-140 promotes adipocyte lineage commitment of C3H10T1/2 pluripotent stem cells via targeting osteopetrosis-associated transmembrane protein 1. J Biol Chem 2013; 288: 8222–8230.
Acknowledgements
We are grateful to all the participants for their invaluable contributions to the study. The Obesity Research Unit team and the staff at the Finnish Twin Cohort Study are acknowledged for their help in the collection of the data. We also thank Dr Milla Kibble for providing critical comments on the manuscript. This study was supported by the Academy of Finland (grants 307339, 251316, 259926, 297908, 266286, 265240 and 263278), the Sigrid Juselius Foundation, the University of Helsinki Research Funds, the Helsinki University Hospital Research Funds, the Paulo Foundation, the Finnish Cultural Foundation Southwest Finland Regional Fund, the Turku University Hospital Research Funds, the Academy of Finland Centre of Excellence in Research on Mitochondria, Metabolism and Disease (grant 272376), grants from Novo Nordisk Foundation, the Biomedicum Helsinki Foundation, the Finnish Diabetes Research Foundation, the Jalmari and Rauha Ahokas Foundation, the Finnish Foundation for Cardiovascular Research, EPITRAIN-FP7-PEOPLE-2012-ITN (grant 316758) and Doctoral Programme in Population Health, University of Helsinki. We are thankful to the Biomedicum Functional Genomics Unit ((FuGU), Helsinki, Finland) and the Microarray Consortium (Oslo, Norway) for running gene expression and DNA methylation microarrays, respectively.
Author contributions
KHP, AR and MO conceived and designed the study. KHP collected the biological samples and performed the clinical investigations. KHP and MO coordinated the study. SB and MO wrote the manuscript. KHP helped in drafting the manuscript and critically commented on it. SB performed the bioinformatics and the statistical analysis of the methylation and expression data and the clinical measurements. SK and KAV assisted in clinical examinations of the participants. SK and SH helped in collecting the samples and extracted the adipocytes. KAV was responsible for the functional imaging studies. JK was responsible for the twin cohort data collection, a subset of which served as the validation cohort. All authors participated in discussions related to analysis and interpretation and have read and approved the final manuscript. MO is the guarantor of this work and as such had full access to the data and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Bollepalli, S., Kaye, S., Heinonen, S. et al. Subcutaneous adipose tissue gene expression and DNA methylation respond to both short- and long-term weight loss. Int J Obes 42, 412–423 (2018). https://doi.org/10.1038/ijo.2017.245
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.245
This article is cited by
-
Epigenetic regulation in metabolic diseases: mechanisms and advances in clinical study
Signal Transduction and Targeted Therapy (2023)
-
Identification of hub genes and candidate herbal treatment in obesity through integrated bioinformatic analysis and reverse network pharmacology
Scientific Reports (2022)
-
Equivalent DNA methylation variation between monozygotic co-twins and unrelated individuals reveals universal epigenetic inter-individual dissimilarity
Genome Biology (2021)
-
DNA methylation signature in blood mirrors successful weight-loss during lifestyle interventions: the CENTRAL trial
Genome Medicine (2020)
-
Mechanisms of weight regain after weight loss — the role of adipose tissue
Nature Reviews Endocrinology (2019)