Abstract
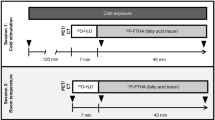
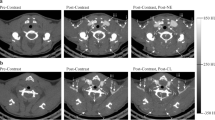
Brown adipose tissue (BAT) has been proposed as a potential target tissue against obesity and its related metabolic complications. Although the molecular and functional characteristics of BAT have been intensively studied in rodents, only a few studies have used human BAT specimens due to the difficulty of sampling human BAT deposits. We established a novel positron emission tomography and computed tomography-guided Bergström needle biopsy technique to acquire human BAT specimens from the supraclavicular area in human subjects. Forty-three biopsies were performed on 23 participants. The procedure was tolerated well by the majority of participants. No major complications were noted. Numbness (9.6%) and hematoma (2.3%) were the two minor complications noted, which fully resolved. Thus, the proposed biopsy technique can be considered safe with only minimal risk of adverse events. Adoption of the proposed method is expected to increase the sampling of the supraclavicular BAT depot for research purposes so as to augment the scientific knowledge of the biology of human BAT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009; 360: 1518–1525.
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360: 1500–1508.
Nedergaard J, Bengtsson T, Cannon B . Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007; 293: E444–E452.
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–1517.
Sidossis L, Kajimura S . Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest 2015; 125: 478–486.
Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 2013; 19: 635–639.
Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 2012; 7: e49452.
Lee P, Greenfield JR, Ho KK, Fulham MJ . A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2010; 299: E601–E606.
Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 2011; 14: 272–279.
Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014; 63: 4089–4099.
Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150: 366–376.
Hu HH, Tovar JP, Pavlova Z, Smith ML, Gilsanz V . Unequivocal identification of brown adipose tissue in a human infant. J Magn Reson Imaging 2012; 35: 938–942.
Lee P, Zhao JT, Swarbrick MM, Gracie G, Bova R, Greenfield JR et al. High prevalence of brown adipose tissue in adult humans. J Clin Endocrinol Metab. 2011; 96: 2450–2455.
Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M et al. Evidence for two types of brown adipose tissue in humans. Nat Med 2013; 19: 631–634.
Shinoda K, Luijten IH, Hasegawa Y, Hong H, Sonne SB, Kim M et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med 2015; 21: 389–394.
Tarnopolsky MA, Pearce E, Smith K, Lach B . Suction-modified Bergstrom muscle biopsy technique: experience with 13,500 procedures. Muscle Nerve. 2011; 43: 717–725.
Gupta S, Wallace MJ, Cardella JF, Kundu S, Miller DL, Rose SC . Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol 2010; 21: 969–975.
Porter C, Reidy PT, Bhattarai N, Sidossis LS, Rasmussen BB . Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc 2014.
Cox RA, Mlcak RP, Chinkes DL, Jacob S, Enkhbaatar P, Jaso J et al. Upper airway mucus deposition in lung tissue of burn trauma victims. Shock 2008; 29: 356–361.
Nicholls DG . Hamster brown-adipose-tissue mitochondria. The control of respiration and the proton electrochemical potential gradient by possible physiological effectors of the proton conductance of the inner membrane. Eur J Biochem 1974; 49: 573–583.
Acknowledgements
The authors want to thank the study participants and the nursing and administrative personnel at the Institute of Translational Sciences Clinical Research Center at the University of Texas Medical Branch and the Metabolism Unit, Shriners Hospital for Children, Galveston, TX, USA. We thank Sarah Toombs Smith of the Sealy Center on Aging, University of Texas Medical Branch, for manuscript editing; Sebastien M Labbé for generating the PET/CT images; Nicholas M Hurren for taking the pictures during the biopsy procedure; Rajesh Kumar of the Department of Nuclear Medicine, University of Texas Medical Branch, for performing the PET/CT scans. This study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences, National Institutes of Health; the American Diabetes Association (1-14-TS-35 to LSS); Shriners Hospitals for Children (grants 84090 and 85310 to LSS); the John Sealy Memorial Endowment Fund for Biomedical Research (grant 66992 to LSS); Ajinomoto Co., Inc. (grant 445800 to LSS) and the Sealy Center on Aging (grant to LSS). MC is funded by the Onassis Foundation. CP is supported, in part, by a National Institute of Disability and Rehabilitation Research Postdoctoral Training Grant, National Institutes of Health (H133P110012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chondronikola, M., Annamalai, P., Chao, T. et al. A percutaneous needle biopsy technique for sampling the supraclavicular brown adipose tissue depot of humans. Int J Obes 39, 1561–1564 (2015). https://doi.org/10.1038/ijo.2015.76
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.76
This article is cited by
-
ADRA1A–Gαq signalling potentiates adipocyte thermogenesis through CKB and TNAP
Nature Metabolism (2022)
-
A new approach to study the sex differences in adipose tissue
Journal of Biomedical Science (2018)