Abstract
Background:
Chronic inflammation in adipose tissue together with obesity induces insulin resistance. Inhibitors of chronic inflammation in adipose tissue can be a potent candidate for the treatment of diabetes; however, only a few compounds have been discovered so far. The objective of this study was to find a novel inhibitor that can suppress the inflammatory response in adipose tissue and to elucidate the intracellular signaling mechanisms of the compound.
Methods:
To find the active compounds, we established an assay system to evaluate the inhibition of induced MCP-1 production in adipocyte/macrophage coculture in a plant extract library. The active compound was isolated by performing high-performance liquid chromatography (HPLC) and was determined as 4β-hydroxywithanolide E (4βHWE) by nuclear magnetic resonance (NMR) and mass spectroscopy (MS) spectral analyses. The effect of 4βHWE on inflammation in adipose tissue was assessed with adipocyte culture and db/db mice.
Results:
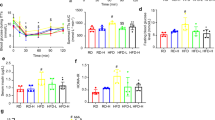
During the screening process, Physalis pruinosa calyx extract was found to inhibit production of MCP-1 in coculture strongly. 4βHWE belongs to the withanolide family of compounds, and it has the strongest MCP-1 production inhibitory effect and lowest toxicity than any other withanolides in coculture. Its anti-inflammatory effect was partially dependent on the attenuation of NF-κB signaling in adipocyte. Moreover, in vivo experiments showed that the oral administration of 4βHWE to db/db mice resulted in the inhibition of macrophage invasion and cytokine expression in adipose tissue after 2 weeks of treatment; improved the plasma adiponectin, non-esterified fatty acids and MCP-1 concentrations; and increased glucose tolerance after 3 to 4 weeks of treatment.
Conclusions:
These results suggest that 4βHWE has anti-inflammatory effect via inhibition of NF-κB activation in adipocyte. Moreover, the attenuation of inflammation in adipocyte has an effect on the inhibition of macrophage accumulation in obese adipose tissue. Consequently, 4βHWE improves impaired glucose tolerance. Thus, 4βHWE is a useful natural anti-inflammatory compound to attenuate progression of diabetes and obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Odegaard JI, Chawla A . Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab 2008; 4: 619–626.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr . Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig 2003; 112: 1796–1808.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Investig 2003; 112: 1821–1830.
Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005; 11: 191–198.
De Taeye BM, Novitskaya T, McGuinness OP, Gleaves L, Medda M, Covington JW et al. Macrophage TNF-alpha contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am J Physiol Endocrinol Metab 2007; 293: E713–E725.
Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab 2007; 6: 386–397.
Verschuren L, Kooistra T, Bernhagen J, Voshol PJ, Ouwens DM, van Erk M et al. MIF deficiency reduces chronic inflammation in white adipose tissue and impairs the development of insulin resistance, glucose intolerance, and associated atherosclerotic disease. Circ Res 2009; 105: 99–107.
Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Investig 2006; 116: 1494–1505.
Vieira AT, Pinho V, Lepsch LB, Scavone C, Ribeiro IM, Tomassini T et al. Mechanisms of the anti-inflammatory effects of the natural secosteroids physalins in a model of intestinal ischaemia and reperfusion injury. Br J Pharmacol 2005; 146: 244–251.
Jacobo-Herrera NJ, Bremner P, Marquez N, Gupta MP, Gibbons S, Munoz E et al. Physalins from Witheringia solanacea as modulators of the NF-kappaB cascade. J Nat Products 2006; 69: 328–331.
Lee CC, Houghton P . Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J Ethnopharmacol 2005; 100: 237–243.
Kizelsztein P, Govorko D, Komarnytsky S, Evans A, Wang Z, Cefalu WT et al. 20-Hydroxyecdysone decreases weight and hyperglycemia in a diet-induced obesity mice model. Am J Physiol Endocrinol Metab 2009; 296: E433–E439.
Maurya R, Akanksha Jayendra, Singh AB, Srivastava AK . Coagulanolide, a withanolide from Withania coagulans fruits and antihyperglycemic activity. Bio-org Med Chem Lett 2008; 18: 6534–6537.
Xu A, Wang H, Hoo RL, Sweeney G, Vanhoutte PM, Wang Y et al. Selective elevation of adiponectin production by the natural compounds derived from a medicinal herb alleviates insulin resistance and glucose intolerance in obese mice. Endocrinology 2009; 150: 625–633.
Lu M, Patsouris D, Li P, Flores-Riveros J, Frincke JM, Watkins S et al. A new antidiabetic compound attenuates inflammation and insulin resistance in Zucker diabetic fatty rats. Am J Physiol Endocrinol Metab 2010; 298: E1036–E1048.
Kaileh M, Vanden Berghe W, Heyerick A, Horion J, Piette J, Libert C et al. Withaferin a strongly elicits IkappaB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J Biol Chem 2007; 282: 4253–4264.
Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chendil D . Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res 2007; 67: 246–253.
Falsey RR, Marron MT, Gunaherath GM, Shirahatti N, Mahadevan D, Gunatilaka AA et al. Actin microfilament aggregation induced by withaferin A is mediated by annexin II. Nat Chem Biol 2006; 2: 33–38.
Bargagna-Mohan P, Hamza A, Kim YE, Khuan Abby Ho Y, Mor-Vaknin N, Wendschlag N et al. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem Biol 2007; 14: 623–634.
Giri S, Rattan R, Haq E, Khan M, Yasmin R, Won JS et al. AICAR inhibits adipocyte differentiation in 3T3L1 and restores metabolic alterations in diet-induced obesity mice model. Nutrition Metab 2006; 3: 31.
Suganami T, Nishida J, Ogawa Y . A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol 2005; 25: 2062–2068.
Toyoda T, Kamei Y, Kato H, Sugita S, Takeya M, Suganami T et al. Effect of peroxisome proliferator-activated receptor-alpha ligands in the interaction between adipocytes and macrophages in obese adipose tissue. Obesity 2008; 16: 1199–1207.
Vessal M, Mehrani HA, Omrani GH . Effects of an aqueous extract of Physalis alkekengi fruit on estrus cycle, reproduction and uterine creatine kinase BB-isozyme in rats. J Ethnopharmacol 1991; 34: 69–78.
Vessal M, Mostafavi-Pour Z, Kooshesh F . Age and sex dependence of the effects of an aqueous extract of Physalis alkekengi fruits on rat hepatic glucose 6-P dehydrogenase activity. Comp Biochem Physiol Part B, Biochem Mol Biol 1995; 111: 675–680.
Wu SJ, Ng LT, Chen CH, Lin DL, Wang SS, Lin CC . Antihepatoma activity of Physalis angulata and P. peruviana extracts and their effects on apoptosis in human Hep G2 cells. Life Sci 2004; 74: 2061–2073.
Quispe-Mauricio A, Callacondo D, Rojas J, Zavala D, Posso M, Vaisberg A . [Cytotoxic effect of physalis peruviana in cell culture of colorectal and prostate cancer and chronic myeloid leukemia]. Revista de gastroenterologia del Peru 2009; 29: 239–246.
Arun M, Asha VV . Preliminary studies on antihepatotoxic effect of Physalis peruviana Linn. (Solanaceae) against carbon tetrachloride induced acute liver injury in rats. J Ethnopharmacol 2007; 111: 110–114.
Bastos GN, Silveira AJ, Salgado CG, Picanco-Diniz DL, do Nascimento JL . Physalis angulata extract exerts anti-inflammatory effects in rats by inhibiting different pathways. J Ethnopharmacol 2008; 118: 246–251.
Martinez W, Ospina LF, Granados D, Delgado G . In vitro studies on the relationship between the anti-inflammatory activity of Physalis peruviana extracts and the phagocytic process. Immunopharmacol Immunotoxicol 2010; 32: 63–73.
Kang H, Kwon SR, Choi HY . Inhibitory effect of Physalis alkekengi L. var. franchetii extract and its chloroform fraction on LPS or LPS/IFN-gamma-stimulated inflammatory response in peritoneal macrophages. J Ethnopharmacol 2011; 135: 95–101.
Nakano D, Ishitsuka K, Hatsuse T, Tsuchihashi R, Okawa M, Okabe H et al. Screening of promising chemotherapeutic candidates against human adult T-cell leukemia/lymphoma from plants: active principles from Physalis pruinosa and structure-activity relationships with withanolides. J Nat Med 2011; 65: 559–567.
Gunasekera SP, Cordell GA, Farnsworth NR . Plant Anticancer Agents XX.* Constituents of Nicandra physalodes. Planta Med 1981; 43: 389–391.
Cordero CP, Morantes SJ, Paez A, Rincon J, Aristizabal FA . Cytotoxicity of withanolides isolated from Acnistus arborescens. Fitoterapia 2009; 80: 364–368.
Budhiraja RD, Sudhir S, Garg KN . Anti-inflammatory activity of 3 beta-hydroxy-2,3-dihydro-withanolide F. Planta Med 1984; 50: 134–136.
Kuboyama T, Tohda C, Komatsu K . Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br J Pharmacol 2005; 144: 961–971.
Kuboyama T, Tohda C, Zhao J, Nakamura N, Hattori M, Komatsu K . Axon- or dendrite-predominant outgrowth induced by constituents from Ashwagandha. Neuroreport 2002; 13: 1715–1720.
Sakurai K, Ishii H, Kobayashi S, Iwao T . Isolation of 4 beta-hydroxywithanolide E, a new withanolide from Physalis peruviana L. Chem Pharm Bull 1976; 24: 1403–1405.
Yen CY, Chiu CC, Chang FR, Chen JY, Hwang CC, Hseu YC et al. 4beta-Hydroxywithanolide E from Physalis peruviana (golden berry) inhibits growth of human lung cancer cells through DNA damage, apoptosis and G2/M arrest. BMC cancer 2010; 10: 46.
Hsieh PW, Huang ZY, Chen JH, Chang FR, Wu CC, Yang YL et al. Cytotoxic withanolides from Tubocapsicum anomalum. J Nat Products 2007; 70: 747–753.
Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K et al. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol 1994; 153: 2052–2063.
Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV . Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med 1990; 171: 35–47.
Libermann TA, Baltimore D . Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol 1990; 10: 2327–2334.
Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 2001; 293: 1673–1677.
Kamon J, Yamauchi T, Muto S, Takekawa S, Ito Y, Hada Y et al. A novel IKKbeta inhibitor stimulates adiponectin levels and ameliorates obesity-linked insulin resistance. Biochem Biophys Res Commun 2004; 323: 242–248.
Weisberg SP, Leibel R, Tortoriello DV . Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 2008; 149: 3549–3558.
Turnbaugh PJ, Backhed F, Fulton L, Gordon JI . Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008; 3: 213–223.
Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012; 488: 178–184.
Acknowledgements
We thank Hiroaki Kisaka and Ryuji Sugiyama for supplying the plant extract library and the Physalis calyx from Kamikoani, Shin Harumatsu for providing technical assistance and Reika Nakagawa for the screening and identification of active fractions with the coculture assay. We also thank Naoko Akimoto for her assistance with 4βHWE purification.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
T Takimoto, Y Kanbayashi, T Toyoda, Y Adachi, C Furuta, K Suzuki, T Miwa and M Bannai are employees of Ajinomoto.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Takimoto, T., Kanbayashi, Y., Toyoda, T. et al. 4β-Hydroxywithanolide E isolated from Physalis pruinosa calyx decreases inflammatory responses by inhibiting the NF-κB signaling in diabetic mouse adipose tissue. Int J Obes 38, 1432–1439 (2014). https://doi.org/10.1038/ijo.2014.33
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2014.33
Keywords
This article is cited by
-
Golden berry 4β-hydroxywithanolide E prevents tumor necrosis factor α-induced procoagulant activity with enhanced cytotoxicity against human lung cancer cells
Scientific Reports (2021)
-
4β-Hydroxywithanolide E and withanolide E from Physalis peruviana L. inhibit adipocyte differentiation of 3T3-L1 cells through modulation of mitotic clonal expansion
Journal of Natural Medicines (2021)
-
A review of nutritional properties and health benefits of Physalis species
Plant Foods for Human Nutrition (2020)
-
Sodium butyrate alleviates adipocyte inflammation by inhibiting NLRP3 pathway
Scientific Reports (2015)