Abstract
Background:
Measurement of cholesterol and triglyceride (TG) fractions in blood has become standard practice in the early detection of atherosclerotic disease pathways. Considerable attention is given nowadays to the presence of these risk factors in children and to start preventive campaigns early in life. In this context, it is imperative to have valid comparative frameworks for interpretation of lipid levels. The aim of this study is to present sex- and age-specific reference values on blood lipid levels in European children aged 2.0–10.9 years.
Methods:
Fasting blood was obtained via either venipuncture or capillary sampling. In 13 579 European non-obese children (50.3% boys), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), TG and TC/HDL-C ratio levels were measured with a point-of-care analyser (Cholestech). Sex- and age-specific reference values were computed with the GAMLSS method with the statistical software R.
Results:
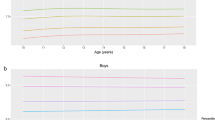
Reference curves and 1st, 3rd, 10th, 25th, 50th, 75th, 90th, 97th and 99th percentile values are presented. HDL-C showed a positive trend with age, from 2 years onwards, but was relatively stable above the age of 7. For LDL-C and TC, linear but small age-related trends were seen. The TC/HDL-C values showed a gradual negative trend from the age of 2 up to 6 and were relatively stable afterwards. For TG, no age trend was found (P=0.285). Boys had higher mean HDL-C values than girls (1.414 vs 1.368 mmol l−1), and lower TC, LDL-C, TC/HDL-C and TG values (3.981 vs 4.087 mmol l−1; 2.297 vs 2.435 mmol l−1; 2.84 vs 3.01mmol l−1; and 0.509 vs 0.542 mmol l−1, respectively).
Conclusions:
These new and recent references could serve as a European orientation of blood lipid values in children in the context of standard medical practice and for the purpose of public health screening.
Similar content being viewed by others
Introduction
In the biomedical literature, the term ‘blood lipids’ most commonly refers to the circulating fractions of cholesterol and triglycerides (TG), biomolecules that are insoluble in water and are therefore embedded in larger structures with a water soluble protein backbone—these structures in the plasma are called lipoproteins. Lipoproteins are further classified in several subcategories, mainly on the basis of their composition and density.1 The main lipid subcategories are very-low-density lipoproteins, low-density lipoproteins (LDL), intermediate-density lipoproteins and high-density lipoproteins (HDL). Triglycerides are structural part of all lipoproteins and are often treated as a separate category in the context of medical laboratory research.
Lipoprotein and triglyceride concentrations in blood are a reflection of the fat metabolism in the human body and are known to be modulated by genetic background and environmental determinants—like diet, physical activity, alcohol use, smoking and so on. Measurement of these lipid fractions in blood has become standard medical and public health practice for early detection of disease pathways involving hereditary- and/or lifestyle-induced disturbances of lipid metabolism. Their role in the typical vascular damage leading to atherosclerotic diseases is now fairly well documented2, 3, 4 and also non-atherosclerotic diseases have been related to blood and tissue lipids, like some neurodegenerative diseases and rheumatoid arthritis.5, 6, 7 Special attention for these lipids is now integrated in a broad context of primary disease prevention and promotion of healthy lifestyle.8,9 Indeed, serum lipids have been in the overall picture of the diet-disease paradigm now since more than half a century already. The first reports on the relation between diet, blood lipids and cardiovascular diseases (the initial famous so-called ‘diet-heart hypothesis’) were from the Seven Countries Study.10,11
Blood lipids have gone through a remarkable journey of rescaling and resetting the optimal plasma range for defining their relevance as risk factors. A complementary set of comparative population studies, long-term follow-up studies, clinical studies and in vitro experiments have led to a gradual decrease in what is considered an optimal range of serum lipid levels for disease prevention. Population targets for total cholesterol (TC) and LDL cholesterol (LDL-C) are today set at 4.9 and 3 mmol l−1, respectively.9,12,13
Meanwhile, it became increasingly obvious that this lifestyle–disease association was already operational in the early stages of life and that risk factors established during childhood have a tendency to track into adulthood and to add to the overall risk profile for chronic diseases.14, 15, 16 Considerable attention is therefore given nowadays to the presence of risk factors in children and to start addressing them in preventive campaigns.17
Against this background, it is of course imperative to have valid comparative frameworks for the interpretation of individual values and population distributions of risk markers like blood lipids. Therefore, the aim of this study is to present sex- and age-specific reference values on blood lipid levels (HDL cholesterol (HDL-C), LDL-C, TC, triglycerides (TG), TC/HDL-C) in European children aged 2.0–10.9 years.
Subjects and methods
Study subjects
A cohort of 16 228 children aged 2–9 years was examined in a population-based baseline survey in eight countries geographically distributed across Europe from North to South and from East to West (Sweden, Germany, Hungary, Italy, Cyprus, Spain, Belgium and Estonia). Standardised instruments and protocols were used for a large battery of measurements done in a period between autumn 2007 and spring 2008.18
The study was not designed to provide a representative sample for each country. Rather, the baseline survey (T0) was the starting point for a longitudinal scientific project based on the largest European children’s cohort established to date.18 This cohort and an additional 2517 children aged 2.0–10.9 years who were newly recruited during a second (first follow-up) survey (T1) 2 years later constitute the actual study population for the present analysis. Exactly the same survey modules were deployed at baseline (T0) and at follow-up (T1).
All children in the defined age group who resided in the selected regions and who attended the primary schools (grades 1 and 2), pre-schools or kindergartens were eligible for participation. Children were approached via schools and kindergartens to facilitate uniform enrolment across all social classes. In addition to the signed informed consent given by parents, each child was asked to give verbal assent immediately before examination. Participants were free to volunteer or renounce for specific modules like, for instance, the blood drawing.
Children who were ill at the time of visit were excluded for blood withdrawal. Valid blood lipid data were eventually available for 14 673 children. Of these children, 1094 obese children were excluded from the analyses to reflect as much as possible the biological variation in a disease-free population, resulting in a final study population of 13 579 children for establishing the reference value database. Outliers have not been excluded. The flow chart in Figure 1 summarises the exclusion steps. The analysis group did not differ in sex distribution from the excluded sample, but the included children were older (6.4 vs 5.9 years, P<0.001).
Body mass index
The children were measured barefooted in underwear and/or T-shirt. Weight was measured in fasting status with an electronic scale (TANITA BC 420 SMA, Tanita Europe GmbH, Sindelfingen, Germany) to the nearest 0.1 kg. Height was measured with a telescopic height measuring instrument (seca 225, stadiometer, seca, Birmingham, UK) to the nearest 0.1 cm. The BMI (body mass index) was obtained as follows: BMI=weight (kg)/height (m)2. Children were classified as obese according to the new International Obesity Task Force criteria using BMI centiles (98.9th centile for obesity in boys, 98.6th centile for obesity in girls).19
Blood collection
Fasting blood was obtained from all children via either venipuncture (65.1% of the children) or capillary sampling (34.9%). It was anticipated that a sizeable number of children would refuse the venipuncture even with local anaesthesia, for example, lidocaïne-prilocaïne (EMLA, AstraZeneca, London, UK) patches provided. Significantly higher levels of TC, HDL-C and LDL-C and significantly lower levels of TG were found in the venous samples vs the capillary samples (all P<0.001).
To ensure that at least basic data on metabolic risk markers were available for as many children as possible, a so-called ‘point-of-care analyser’ was used for both the capillary and the venous blood samples. This technique—Cholestech LDX (Cholestech, Hayward, CA, USA)—allowed to assess on the spot values for blood glucose, HDL-C and TC and TG using one drop of either capillary blood from the fingertip or taken from the serum tube (http://www.cholestech.com/products/ldx_overview.htm). This portable device has been validated for lipid analyses against standard laboratory methods in adults (r between 0.91 and 0.99; no significant mean bias)20 and the system is certified by the Centers for Disease Control cholesterol reference method laboratory network. The detection ranges are: TC range 2.6–12.9 mmol l−1; TG range 0.50–7.31 mmol l−1; and HDL-C range 0.39–2.62 mmol l−1. LDL-C was not measured directly through this technique, but calculated using the Friedewald formula LDL-C=TC−HDL-C−TG/5.0 (mg dl−1) based on the Cholestech results.21
Quality management
All measurements followed detailed standard operating procedures that were laid down in the general survey manual and were finalised after the pre-test of all survey modules. Field personnel from each study centre participated in central training and organised local training sessions thereafter. The coordinating centre conducted site visits to each study location during field surveys to check adherence of field staff to the standard operating procedures. All study centres used the same technical equipment that was purchased centrally to maximise comparability of data. Databases and computer-assisted questionnaires included automated plausibility checks. All numerical variables were entered twice independently. Inconsistencies identified by additional plausibility checks were rectified by the study centres. To further check for the quality of data, sub-samples of study subjects were examined repeatedly to calculate the inter- and intra-observer reliability of anthropometric measurements.22,23
Statistical analysis
We calculated percentile curves of blood lipids (HDL-C, LDL-C, TC, TG, TC/HDL-C) as a function of the covariate age stratified by sex using the General Additive Model for Location Scale and Shape (GAMLSS) as extension of the least mean squares method. The least mean squares method models three parameters: the skewness (L) accounts for the deviation from a normal distribution using a Box–Cox transformation (Box–Cox t), the median (M) models the outcome variable depending on one explanatory variable and the coefficient of variation (S) accounts for the variation of data points around the mean and adjusts for non-uniform dispersion. The GAMLSS is able to model more than one covariate and also other distributions. We used the GAMLSS package (version 4.2–6) of the statistical software R (version 3.0.1, www.cran.r-project.org).24 Different distributions, that is, the logistic, normal, inverse Gaussian, Box–Cox t, Box–Cox power exponential and Box–Cox Cole and Green, were fitted to the observed distribution of blood lipids. Moreover, the influence of age on parameters of the considered distributions was modelled either as a constant, as a linear function, or as a cubic spline of the covariate. Goodness of fit was assessed by the Bayesian Information Criterion and Q-Q plots to select the final model including the fitted distribution of blood lipids and the influence of covariates on distribution parameters. Worm plots were used as a diagnostic tool to assess whether adjustment for kurtosis was required.25 Finally, percentile curves for the 1st, 3rd, 10th, 25th, 50th, 75th, 90th, 97th and 99th percentiles were calculated based on the model that showed the best goodness of fit.24,26 Sensitivity analyses were executed to assess the effect of excluding obese children and capillary blood samples.
Results
Table 1 describes the analysis group of 13 579 non-obese European children, 50.3% boys.
Significant sex differences were found for all blood lipid variables (P<0.001): HDL-C values were higher in boys and TC, LDL-C, TC/HDL-C and TG were higher in girls. Significant correlations with age were found for all cholesterol values (P<0.001): an age-related positive trend for TC and HDL-C but an age-related negative trend for LDL-C and TC/HDL-C. For TG, no significant correlation with age was detected (P=0.285). Consequently, age non-specific reference values are given for TG. In this context, it should be noted that the TG levels were often below the detection limit of 0.50 mmol l−1.
Apart from age and sex, also seasonal differences were detected. The school visits were executed from the end of September until the beginning of June with a uniform distribution over the months. Analysis of variance analyses showed no difference in TC between the months during the survey period, but HDL-C was highest in April and LDL-C and triglycerides were highest in October, November and December.
The best fit in GAMLSS was retrieved for HDL-C using the Box–Cox power exponential distribution with parameters modelled in both sexes as follows: μ as a cubic spline, log(σ) linearly and the shape parameters ν and log(τ) as constants. For LDL, the best fit was obtained using the Box–Cox t distribution with parameters modelled as follows: μ linearly and log(σ), ν and log(τ) as constants. For TC, using the inverse Gaussian distribution with parameters log(μ) and log(σ) modelled linearly. Finally, for TC/HDL-C, using the Box–Cox t distribution with parameters modelled as follows: μ as a cubic spline, log(σ) linearly and ν and log(τ) as constants.
The reference values for blood lipids are shown in Table 2 for boys and Table 3 for girls. Percentile curves are presented in Figure 2. HDL-C showed a positive age trend up to the age of 7 and was relatively stable afterwards. For LDL-C and TC, linear but small age-related changes were seen. The TC/HDL-C values showed a gradual decrease from the age of 2 up to 6 and were relatively stable afterwards. Sensitivity analyses revealed that the percentile values did not really differ when excluding obese children or when excluding capillary blood samples (Supplementary Figures A and B). In Supplementary Table A of the Supplementary Material, reference values are given for the whole sample without exclusion for obesity.
Discussion
It has been shown convincingly that atherosclerotic changes in the vessel wall often already start during youth in populations that have adopted a modern Western lifestyle with its typical high-fat energy dense diet and sedentary pattern.27 In view of this observation, early screening of blood lipid values in children and adolescents can indeed be warranted from the point of view of cardiovascular disease prevention.28 To do this in a scientifically sound way, the availability of valid data on normal blood lipid distributions is an imperative premise.
In this paper, we present age- and sex-specific reference values for blood TG, TC, LDL-C, HDL-C and TC/HDL-C ratio from a large sample of European children aged 2.0–10.9 years. Percentile curves were calculated with the so-called GAMLSS, developed by Rigby and Stasinopoulos.29 This method is an extension of the least mean squares method to model the distribution of blood lipids depending on age, while accounting for dispersion, skewness and particularly the kurtosis of this distribution.26,29 This GAMLSS is today generally accepted as standard technique for establishing reference curves for biological parameters. At the interpretational level, it allows for visualising nonlinear changes, for example, age-related changes in the parameter of interest (in casu blood lipids). In addition, these nonlinear changes can also be established for non-normal variables, as it is based on nonparametric modelling. To the authors’ knowledge, no previous study has ever established reference values for blood lipids from such a large sample of healthy children from populations within such a wide geographical scope in Europe.
However, although the large numbers and the geographical distribution are clearly important positive points for establishing a set of reference values that could be framed as ‘European’, there are some limitations to be considered as well. As already pointed out in the editorial, the regions included in every IDEFICS (Identification and prevention of Dietary- and lifestyle induced health Effects in Children and InfantS) country were not per se representative for the whole country and this was fully compatible with the original research questions addressed by this European Union-funded project. Nevertheless, there are no obvious reasons in any of the participating countries to assume that regional differences in lifestyle in the selected areas would have been operational at a level that could differentially influence blood lipid values on population level. Both the control and intervention regions in all countries were chosen to be comparable to each other and to the national average as far as classical socioeconomic and demographic indicators are concerned. In addition, a most meticulous standardisation of measurements and analytical circumstances and subsequent procedures for quality control have been successfully implemented in the context of this collaborative endeavour. As a result, we do not present reference values stratified by country.
In the authors’ view, the set of values in this paper can be used as an interpretational framework in clinical and public health practice with a fairly high confidence towards true representation of European biological variability in the different lipid fractions and their evolution as a function of age during early childhood. A major limitation for generalised use in medical practice is the age group, as reference values during pubertal maturation might be even more relevant for universal screenings.
Obese children—as defined by the International Obesity Task Force criteria19—have been omitted from the database used for the analyses in this paper. The reason for this is that excessive fat deposition is documented as positively correlated with blood lipid levels, also in children and adolescents.30,31 As reference values are expected to reflect as much as possible the biological variation in a disease-free population, it was a logical methodological consideration to withdraw obese children from the results, even if sensitivity analyses showed that their inclusion did not significantly change the overall conclusions or reference standards (Supplementary Figure A).
Obviously, there are other factors than disease (for example, lifestyle factors) that can influence blood lipids at the individual level and that can also affect population distributions. However, such influences cannot reasonably be filtered out of the study population in a systematic way as there is no evidence-based framework for understanding at individual level which dose of lifestyles or personal traits would have to be considered as critically influencing blood lipids at this age and would therefore qualify as exclusion criterion. The well-documented problem of familial dyslipidaemias has been left outside the scope of exclusions as well. Although it is appreciated that a relevant proportion of children with familial dyslipidaemia will already present with substantial elevation of LDL-C levels, there is no information available that would allow accurate identification (and subsequent exclusion) of these children.1
It has to be noted that the results for blood lipids are partly based on venous blood analyses and partly based on capillary blood analyses—in both cases however the Cholestech protocol and equipment was used. The analysis of both blood fractions was done immediately after blood withdrawal on the whole-blood fraction. Studies on the influence of the puncture site on the blood lipid levels are scarce but it has been shown that cholesterol measurement in fingertip capillary specimens is in close agreement (within 1.7%) with conventional venous measurement.32 In contrast, however, in our study sample, we found significantly higher levels of TC, HDL-C and LDL-C and significantly lower levels of TG in the venous samples. The mean differences ranged between 0.02 mmol l−1 for HDL-C and 0.07 mmol l−1 for TG. Taken into account that, according to the meta-analysis of Gordon et al.,33 a 0.026 mmol l−1 increase in HDL-C was associated with a significant coronary heart disease risk decrement of 2% in men and 3% in women, these differences might be relevant from a public health point of view. Nevertheless, compared with the broad range of lipid levels between the 5th and the 95th percentile in our age group, it was decided to calculate the reference values on the pooled data. Moreover, sensitivity analyses revealed no large differences in reference curves/values when including the capillary blood samples (Supplementary Figure B).
Geographical and historical comparisons of data sets can be interesting, although they have to be treated with caution in view of potential differences in recruitment, field work conditions and analytical techniques. Also, time trends could exist, for example, a favourable overall trend in serum lipid concentrations was observed among youths in the United States between 1988–1994 and 2007–2010, in contrast, almost 1 in 10 had elevated TC in 2007–2010.34
A large sample of French children at the age of 4 years has been studied on TC levels by Vincelet et al.35 in 2002. Mean values for TC were at 4.5 mmol l−1 in girls and 4.4 mmol l−1 in boys, which is substantially higher than the values found in our European sample.
In contrast with these French findings, an Italian study36 in more than 7000 Italian children from the Lazio region showed a mean TC of 3.5 mmol l−1 in boys and 3.7 mmol l−1 in girls, which is clearly lower than the values in our European children. Although differences in methodology can explain part of these differences, these comparisons show that regional variations in lipid levels are observed within the European region, which are likely to be explained by differences in lifestyle factors, for example, adherence to Mediterranean diet.
In a large sample of Chinese children from Beijing examined in 2006,37 TC mean values were observed between 3.6 and 4.2 mmol l−1 in boys aged 3–9 years and between 3.5 and 4.1 mmol l−1 in girls of this age. For HDL-C, the values were respectively between 1.1 and 1.6 mmol l−1 and between 1.2 and 1.5 mmol l−1. LDL-C ranged between 1.9 and 2.4 mmol l−1 and between 2 and 2.3 mmol l−1. These values are also largely in line with the European observations from our sample. The major difference is that, in our study population, apparently, the sex differences in HDL-C are clearly more marked with higher values on average in boys. Related to this, the sex differences with higher TG values in girls in our study might have clinical relevance, as TG appear to be the main lipid risk factor in women.38,39
In a recent study carried out in Turkey,40 fasting serum lipid values have been reported for disease-free population-based samples of 383 boys and 248 girls in the age group 1–6 years and 522 boys and 521 girls in the age range 7–10 years. Mean values for TC, LDL-C and HDL-C were very similar to the values found in our sample. Remarkably, however, the serum TG values were on average almost double the values found in the IDEFICS cohort (respectively, 1.08, 1.05, 0.96 and 1.08 mmol l−1).
Finally, in boys and girls from the National Health and Nutrition Examination survey carried out between 1988 and 1994 in USA,41 comparable blood lipid values were observed as in the IDEFICS cohort, with National Health and Nutrition Examination survey TC levels varying between 3.3 mmol l−1 in boys aged 7–10 years and 4.3 mmol l−1 in girls aged 7–10 years; for HDL-C, the National Health and Nutrition Examination survey values ranged from 1.3 mmol l−1 in girls aged 3–6 years to 1.4 mmol l−1 in boys aged 7–10 years.
It is worthwhile comparing the data from this cohort of European children with those from another European cohort of adolescents (HELENA42) studied in 2006—hence comparing two European birth cohorts with a time interval of on average around 6 years.
In HELENA, serum lipid values of 1049 European adolescents (12.5–17.5 years) were measured and showed sex differences with consistently higher lipid values (HDL-C, LDL-C, TC and TG) in girls as compared with boys. The same applies to the IDEFICS cohort, except that HDL-C levels were higher in boys. This finding confirms once more that pubertal changes in boys interact with HDL metabolism and are at the origin of the well-known sex divergence in HDL metabolism in adults.
Conclusion
In the authors’ view, these new and recent references may serve as a European orientation of blood lipid values in children in the context of standard medical practice and for the purpose of public health screening.
References
Longo D, Fuacin A, Kasper D, Hauser S, Jameson J, Loscalzo J . Harrison's Principles of Internal Medicine. The McGraw-Hill Companies, Inc: New York, 2012.
Miller YI, Choi SH, Fang L, Tsimikas S . Lipoprotein modification and macrophage uptake: role of pathologic cholesterol transport in atherogenesis. Subcell Biochem 2010; 51: 229–251.
Mureddu GF, Brandimarte F, De Luca L . High-density lipoprotein levels and risk of cardiovascular events: a review. J Cardiovasc Med (Hagerstown) 2012; 13: 575–586.
Shekelle RB, Shryock AM, Paul O, Lepper M, Stamler J, Liu S et al. Diet, serum cholesterol, and death from coronary heart disease. The Western Electric study. N Engl J Med 1981; 304: 65–70.
Adibhatla RM, Hatcher JF . Altered lipid metabolism in brain injury and disorders. Subcell Biochem 2008; 49: 241–268.
Amezaga Urruela M, Suarez-Almazor ME . Lipid paradox in rheumatoid arthritis: changes with rheumatoid arthritis therapies. Curr Rheumatol Rep 2012; 14: 428–437.
Reed TT . Lipid peroxidation and neurodegenerative disease. Free Radic Biol Med 2011; 51: 1302–1319.
Cooper A, Nherera L, Calvert N, O'Flynn N, Turnbull N, Robson J et al. Clinical Guidelines and Evidence Review for Lipid Modification: Cardiovascular Risk Assessment and the Primary and Secondary Prevention of Cardiovascular Disease. National Collaborating Centre for Primary Care and Royal College of General Practitioners: London, UK, 2008.
Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012; 33: 1635–1701.
Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R et al. The diet and 15-year death rate in the seven countries study. Am J Epidemiol 1986; 124: 903–915.
Kromhout D, Menotti A, Bloemberg B, Aravanis C, Blackburn H, Buzina R et al. Dietary saturated and trans fatty acids and cholesterol and 25-year mortality from coronary heart disease: the Seven Countries Study. Prev Med 1995; 24: 308–315.
National Cholesterol Education Program Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143–3421.
Talwalkar PG, Sreenivas CG, Gulati A, Baxi H . Journey in guidelines for lipid management: from adult treatment panel (ATP)-I to ATP-III and what to expect in ATP-IV. Indian J Endocrinol Metab 2013; 17: 628–635.
Camhi SM, Katzmarzyk PT . Tracking of cardiometabolic risk factor clustering from childhood to adulthood. Int J Pediatr Obes 2010; 5: 122–129.
Vik KL, Romundstad P, Nilsen TI . Tracking of cardiovascular risk factors across generations: family linkage within the population-based HUNT study, Norway. J Epidemiol Community Health 2013; 67: 564–570.
Nicklas TA, von Duvillard SP, Berenson GS . Tracking of serum lipids and lipoproteins from childhood to dyslipidemia in adults: the Bogalusa Heart Study. Int J Sports Med 2002; 23: S39–S43.
Bugge A, El-Naaman B, McMurray RG, Froberg K, Andersen LB . Tracking of clustered cardiovascular disease risk factors from childhood to adolescence. Pediatr Res 2013; 73: 245–249.
Ahrens W, Bammann K, Siani A, Buchecker K, De Henauw S, Iacoviello L et al. The IDEFICS cohort: design, characteristics and participation in the baseline survey. Int J Obes 2011; 35: S3–S15.
Cole TJ, Lobstein T . Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes 2012; 7: 284–294.
Shephard MD, Mazzachi BC, Shephard AK . Comparative performance of two point-of-care analysers for lipid testing. Clin Lab 2007; 53: 561–566.
Friedewald WT, Levy RI, Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502.
Marangoni F, Colombo C, Galli C . A method for the direct evaluation of the fatty acid status in a drop of blood from a fingertip in humans: applicability to nutritional and epidemiological studies. Anal Biochem 2004; 326: 267–272.
Stomfai S, Ahrens W, Bammann K, Kovacs E, Marild S, Michels N et al. Intra- and inter-observer reliability in anthropometric measurements in children. Int J Obes (Lond) 2011; 35: S45–S51.
Stasinopoulos DM, Rigby RA . Generalized additive models for location scale and shape (GAMLSS) in R. J Stat Softw 2007; 23: 1–46.
van Buuren S, Fredriks M . Worm plot: a simple diagnostic device for modelling growth reference curves. Stat Med 2001; 20: 1259–1277.
Cole TJ, Stanojevic S, Stocks J, Coates AL, Hankinson JL, Wade AM . Age- and size-related reference ranges: a case study of spirometry through childhood and adulthood. Stat Med 2009; 28: 880–898.
Juonala M, Magnussen CG, Venn A, Dwyer T, Burns TL, Davis PH et al. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood the cardiovascular risk in young finns study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation 2010; 122: 2514–2520.
Kusters DM, Vissers MN, Wiegman A, Kastelein JJP, Hutten BA . Treatment of dyslipidaemia in childhood. Expert Opin Pharmacother 2010; 11: 739–753.
Rigby RA, Stasinopoulos DM . Generalized additive models for location, scale and shape. J Roy Stat Soc C Appl Stat 2005; 54: 507–544.
Reinehr T, Andler W, Denzer C, Siegried W, Mayer H, Wabitsch M . Cardiovascular risk factors in overweight German children and adolescents: relation to gender, age and degree of overweight. Nutr Metab Cardiovas 2005; 15: 181–187.
Tresaco B, Moreno LA, Ruiz JR, Ortega FB, Bueno G, Gonzalez-Gross M et al. Truncal and abdominal fat as determinants of high triglycerides and low HDL-cholesterol in adolescents. Obesity 2009; 17: 1086–1091.
Warnick GR, Leary ET, Ammirati EB, Allen MP . Cholesterol in fingerstick capillary specimens can be equivalent to conventional venous measurements. Arch Pathol Lab Med 1994; 118: 1110–1114.
Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD et al. High-density lipoprotein cholesterol and cardiovascular-disease—4 prospective American-studies. Circulation 1989; 79: 8–15.
Kit BK, Carroll MD, Lacher DA, Sorlie PD, DeJesus JM, Ogden CL . Trends in serum lipids among US youths aged 6 to 19 years, 1988–2010. J Am Med Assoc 2012; 308: 591–600.
Vincelet C, Bruckert E, Le Corff J, Boisson M, Foucault C . The interest of cholesterol levels in young children. Study in a population of 4697 children aged 4. Presse Med 2004; 33: 1417–1420.
Angelico F, Hurtova M, Liuti A, Francioso S, Urbinati G . Cholesterol levels in Italian school children: results of an opportunistic survey. Nutr Metab Cardiovasc Dis 2000; 10: 24–27.
Liao Y, Mi J, Wang Y, Chen S, Liu Y, Tang C et al. Study on the reference values of serum lipids in children aged 3–18 years old in Beijing, China. Pediatr Int 2010; 52: 472–479.
Bengtsson C, Bjorkelund C, Lapidus L, Lissner L . Associations of serum lipid concentrations and obesity with mortality in women: 20 year follow up of participants in prospective population study in Gothenburg, Sweden. BMJ 1993; 307: 1385–1388.
Hokanson JE, Austin MA . Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk 1996; 3: 213–219.
Can M, Piskin E, Guven B, Acikgoz S, Mungan G . Evaluation of serum lipid levels in children. Pediatr Cardiol 2013; 34: 566–569.
Hickman TB, Briefel RR, Carroll MD, Rifkind BM, Cleeman JI, Maurer KR et al. Distributions and trends of serum lipid levels among United States children and adolescents ages 4–19 years: Data from the third National Health and Nutrition Examination Survey. Prev Med 1998; 27: 879–890.
Spinneker A, Egert S, Gonzalez-Gross M, Breidenassel C, Albers U, Stoffel-Wagner B et al. Lipid, lipoprotein and apolipoprotein profiles in European adolescents and its associations with gender, biological maturity and body fat—the HELENA Study. Eur J Clin Nutr 2012; 66: 727–735.
Acknowledgements
This work was done as part of the IDEFICS Study (www.idefics.eu). We gratefully acknowledge the financial support of the European Community within the Sixth RTD Framework Programme Contract No. 016181 (FOOD). The authors are also grateful to the Volkswagen Foundation that financially supported the production of this supplement. MdL received consulting fees from Barilla & Co. MdL also received grant support from a European Commission (ATHENA) and Barilla & co, through the School of Medicine of Grenoble University.
DISCLAIMER
The information in this document reflects the authors' view and is provided as is.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
De Henauw, S., Michels, N., Vyncke, K. et al. Blood lipids among young children in Europe: results from the European IDEFICS study. Int J Obes 38 (Suppl 2), S67–S75 (2014). https://doi.org/10.1038/ijo.2014.137
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2014.137
This article is cited by
-
Tracking of serum lipids in healthy children on a year-to-year basis
BMC Cardiovascular Disorders (2023)
-
Maternal lipid levels in early pregnancy as a predictor of childhood lipid levels: a prospective cohort study
BMC Pregnancy and Childbirth (2022)
-
Prevalence and factors associated with dyslipidemia among adolescents in Saudi Arabia
Scientific Reports (2022)
-
Sodium assessment in neonates, infants, and children: a systematic review
European Journal of Pediatrics (2022)
-
Media use trajectories and risk of metabolic syndrome in European children and adolescents: the IDEFICS/I.Family cohort
International Journal of Behavioral Nutrition and Physical Activity (2021)