Abstract
Background/Objectives:
In animal studies, exposure to multivitamins may be associated with obesity in the offspring; however, data in humans are sparse. We therefore examined the association between prenatal vitamin intake during pregnancy and offspring obesity.
Subjects/Methods:
We investigated the association between prenatal vitamin intake and obesity among 29 160 mother–daughter dyads in the Nurses’ Health Study II. Mothers of participants provided information on prenatal vitamin use during pregnancy with the nurse daughter. Information on body fatness at ages 5 and 10, body mass index (BMI) at age 18, weight in 1989 and 2009, waist circumference, and height was obtained from the daughter. Polytomous logistic regression was used to predict BMI in early adulthood and adulthood, and body fatness in childhood. Linear regression was used to predict waist circumference in adulthood.
Results:
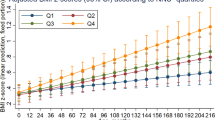
In utero exposure to prenatal vitamins was not associated with body fatness, either in childhood or in adulthood. Women whose mothers took prenatal vitamins during pregnancy had a covariate-adjusted odds ratio (OR) of being obese in adulthood of 0.99 (95% confidence interval (CI) 0.92–1.05, P-value=0.68) compared with women whose mothers did not take prenatal vitamins. Women whose mothers took prenatal vitamins during pregnancy had a covariate-adjusted OR of having the largest body shape at age 5 of 1.02 (95% CI 0.90–1.15, P-value=0.78). In additional analyses, in utero exposure to prenatal vitamins was also unrelated to adult abdominal adiposity.
Conclusions:
Exposure to prenatal vitamins was not associated with body fatness either in childhood or in adulthood.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ogden CL, Carroll MD, Kit BK, Flegal KM . Prevalence of obesity in the United States 2009-2010. NCHS data brief, no 82. 2012 National Center for Health Statistics: Hyattsville, MD, 2012.
National Center for Health Statistics Prevalence of overweight, obesity and extreme obesity among adults: United States, trends 1976-80 through 2005-2006. 2008.
Ogden CL, Carroll MD Prevalence of Obesity Among Children and Adolescents: United States, Trends 1963–1965 through 2007–2008. 2010.
Flegal KM, Graubard BI, Williamson DF, Gail MH . Cause-Specific Excess Deaths Associated With Underweight, Overweight, and Obesity. JAMA 2007; 298: 2028–2037.
Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One 2013; 8: 1.
Fujihara S, Mori H, Kobara H, Nishiyama N, Kobayashi M, Oryu M et al. Metabolic syndrome, obesity, and gastrointestinal cancer. Gastroenterol Res Pract 2012; 2012: 483623.
Reeves KW, Carter GC, Rodabough RJ, Lane D, McNeeley SG, Stefanick ML et al. Obesity in relation to endometrial cancer risk and disease characteristics in the Women's Health Initiative. Gynecol Oncol 2011; 121: 376–382.
Gesink Law DC, Maclehose RF, Longnecker MP . Obesity and time to pregnancy. Hum Reprod 2007; 22: 414–420.
Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sørensen TIA, Olsen J . Subfecundity in overweight and obese couples. Hum Reprod 2007; 22: 1634–1637.
Chen Y, Jiang Y, Mao Y . Association between obesity and depression in Canadians. J Womens Health 2009; 18: 1687–1692.
Cai LP, Lubitz JM, Flegal KMP, Pamuk ERP . The Predicted Effects of Chronic Obesity in Middle Age on Medicare Costs and Mortality. Med Care 2010; 48: 510–517.
Barker DJ, Osmond C . Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986; 1: 1077–1081.
Ravelli GP, Stein ZA, Susser MW . Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976; 295: 349–353.
Lamb MM, Dabelea D, Yin X, Ogden LG, Klingensmith GJ, Rewers M et al. Early-Life Predictors of Higher Body Mass Index in Healthy Children. Ann Nutr Met 2010; 56: 16–22.
Stuebe AM, Forman M, Michels K . Maternal-recalled gestational weight gain, pre-pregnancy body mass index, and obesity in the daughter. Int J Obes 2009; 33: 743–752.
Schack-Nielsen L, Michaelsen KF, Gamborg M, Mortensen EL, Sorensen TIA . Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int J Obes 2009; 34: 67–74.
Heerwagen MJR, Miller MR, Barbour LA, Friedman JE . Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol 2010; 299: R711–R722.
Szeto IMY, Das PJ, Aziz A, Anderson GH . Multivitamin supplementation of Wistar rats during pregnancy accelerates the development of obesity in offspring fed an obesogenic diet. Int J Obes 2009; 33: 364–372.
Michels KB, Willett WC, Graubard BI, Vaidya RL, Cantwell MM, Sansbury LB et al. A longitudinal study of infant feeding and obesity throughout life course. Int J Obes 2007; 31: 1078–1085.
Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC . The validity of recalled weight among younger women. Int J Obes Relat Metab Disord 1995; 19: 570–572.
Stunkard AJ, Sørensen T, Schulsinger F . Use of the Danish Adoption Register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis 1983; 60: 115–120.
Must A, Willett WC, Dietz WH . Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol 1993; 138: 56–64.
Koprowski C, Coates RJ, Bernstein L . Ability of women to recall past body size and age at menarche. Obes Res 2001; 9: 478–485.
Muñoz KA, Ballard-Barbash R, Graubard BI, Swanson CA, Schairer C, Kahle LL . Recall of body weight and body size estimation in women enrolled in the breast cancer detection and demonstration project (BCDDP). Int J Obes Relat Metab Disord 1996; 20: 854–859.
Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol 2002; 155: 672–679.
Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC . Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990; 1: 466–473.
Lewis SJ, Leary S, Davey Smith G, Ness A . Body composition at age 9 years, maternal folate intake during pregnancy and methyltetrahydrofolate reductase (MTHFR) C677T genotype. Br J Nutr 2009; 102: 493–496.
Bouret SG . Role of early hormonal and nutritional experiences in shaping feeding behavior and hypothalamic development. J Nutr 2010; 140: 653–657.
Taylor PD, Poston L . Developmental programming of obesity in mammals. Exp Physiol 2007; 92: 287–298.
Muhlhausler B, Smith SR . Early-life origins of metabolic dysfunction: role of the adipocyte. Trends Endocrinol Metab 2009; 20: 51–57.
Bower C, Stanley J . Dietary folate as a risk factor for neural-tube defects: evidence from a case-control study in Western Australia. Med J Aust 1989; 150: 613–619.
Mills JL, Rhoads GG, Simpson JL, Cunningham GC, Conley MR, Lassman MR et al. The absence of a relation between the periconceptional use of vitamins and neural-tube defects. National Institute of Child Health and Human Development Neural Tube Defects Study Group. N Engl J Med 1989; 321: 430–435.
Mulinare J, Cordero JF, Erickson J, Berry RJ . Periconceptional use of multivitamins and the occurrence of neural tube defects. JAMA 1988; 260: 3141–3145.
Shaw GM, Schaffer D, Velie EM, Morland K, Harris JA . Periconceptional vitamin use, dietary folate, and the occurrence of neural tube defects. Epidemiology 1995; 6: 219–226.
Werler MM, Shapiro S, Mitchell AA . Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA 1993; 269: 1257–1261.
Czeizel AE, Dudás I . Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992; 327: 1832–1835.
MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 1991; 338: 131–137.
Milunsky A, Jick H, Jick SS, Bruell CL, MacLaughlin DS, Rothman KJ et al. Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. JAMA 1989; 262: 2847–2852.
Acknowledgements
All authors designed the study; MMD performed statistical analysis and holds primary responsibility for the final content and drafted the manuscript; and all of the authors contributed intellectual content to the manuscript. All authors read and approved the final manuscript. The Nurses’ Mothers’ Cohort Study was funded by the Intramural Research Program of the National Cancer Institute research contract N02-RC-17027, and by PO 263 MQ 411027 from the National Cancer Institute. The Nurses’ Health Study II is supported by Public Health Service grant CA50385 from the National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dougan, M., Willett, W. & Michels, K. Prenatal vitamin intake during pregnancy and offspring obesity. Int J Obes 39, 69–74 (2015). https://doi.org/10.1038/ijo.2014.107
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2014.107
This article is cited by
-
The Uterine Environment and Childhood Obesity Risk: Mechanisms and Predictions
Current Nutrition Reports (2023)