Abstract
Objective:
To examine independent and combined cross-sectional associations between movement behaviors (physical activity (PA), sedentary time, sleep duration, screen time and sleep disturbance) and fat mass index (FMI), as well as to examine longitudinal associations between movement behaviors and FMI.
Methods:
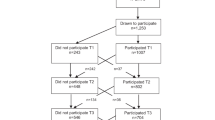
Cross-sectional and longitudinal analyses were done using data from the OPUS school meal study on 785 children (52% boys, 13.4% overweight, ages 8–11 years). Total PA, moderate-to-vigorous PA (MVPA), sedentary time and sleep duration (7 days and 8 nights) were assessed by an accelerometer and FMI was determined by dual-energy X-ray absorptiometry (DXA) on three occasions over 200 days. Demographic characteristics, screen time and sleep disturbance (Children’s Sleep Habits Questionnaire) were also obtained.
Results:
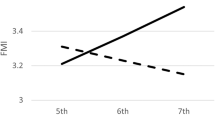
Total PA, MVPA and sleep duration were negatively associated with FMI, while sedentary time and sleep disturbances were positively associated with FMI (P⩽0.01). However, only total PA, MVPA and sleep duration were independently associated with FMI after adjustment for multiple covariates (P<0.001). Nevertheless, combined associations revealed synergistic effects among the different movement behaviors. Changes over time in MVPA were negatively associated with changes in FMI (P<0.001). However, none of the movement behaviors at baseline predicted changes in FMI (P>0.05), but higher FMI at baseline predicted a decrease in total PA and MVPA, and an increase in sedentary time (P⩽0.001), even in normal-weight children (P⩽0.03).
Conclusion:
Total PA, MVPA and sleep duration were independently associated with FMI, and combined associations of movement behaviors showed a synergistic effect with FMI. In the longitudinal study design, a high FMI at baseline was associated with lower PA and higher sedentary time after 200 days but not vice versa, even in normal-weight children. Our results suggest that adiposity is a better predictor of PA and sedentary behavior changes than the other way around.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Riddoch CJ, Leary SD, Ness AR, Blair SN, Deere K, Mattocks C et al. Prospective associations between objective measures of physical activity and fat mass in 12-14 year old children: the Avon Longitudinal Study of Parents and Children (ALSPAC). BMJ 2009; 339: b4544.
Prentice-Dunn H, Prentice-Dunn S . Physical activity, sedentary behavior, and childhood obesity: a review of cross-sectional studies. Psychol Health Med 2012; 17: 255–273.
Must A, Tybor D . Physical activity and sedentary behavior: a review of longitudinal studies of weight and adiposity in youth. Int J Obes 2005; 29: S84–S96.
Carter PJ, Taylor BJ, Williams SM, Taylor RW . Longitudinal analysis of sleep in relation to BMI and body fat in children: the FLAME study. BMJ 2011; 342: d2712.
Jarrin D, McGrath J, Drake C . Beyond sleep duration: distinct sleep dimensions are associated with obesity in children and adolescents. Int J Obes 2013; 37: 552–558.
Janz KF, Burns TL, Levy SM . Tracking of activity and sedentary behaviors in childhood: the Iowa Bone Development Study. Am J Prev Med 2005; 29: 171–178.
Metcalf BS, Hosking J, Jeffery A, Voss L, Henley W, Wilkin T . Fatness leads to inactivity, but inactivity does not lead to fatness: a longitudinal study in children (EarlyBird 45). Arch Dis Child 2011; 96: 942–947.
Damsgaard CT, Dalskov SM, Petersen RA, Sørensen LB, Mølgaard C, Biltoft-Jensen A et al. Design of the OPUS School Meal Study: a randomised controlled trial assessing the impact of serving school meals based on the New Nordic Diet. Scand J Public Health 2012; 0: 1–11.
Trost SG, Loprinzi PD, Moore R, Pfeiffer KA . Comparison of accelerometer cut points for predicting activity intensity in youth. Med Sci Sports Exerc 2011; 43: 1360–1368.
Sadeh A, Sharkey KM, Carskadon MA . Fundamental research activity-based sleep-wake identification: an empirical test of methodological issues. Sleep 1994; 17: 201–207.
Biltoft-Jensen A, Bysted A, Trolle E, Christensen T, Knuthsen P, Damsgaard CT et al. Evaluation of Web-based dietary assessment software for children: comparing reported fruit, juice and vegetable intakes with plasma carotenoid concentration and school lunch observations. Br J Nutr 2012; 27: 1–10.
Biltoft‐Jensen A, Trolle E, Christensen T, Islam N, Andersen L, Egenfeldt‐Nielsen S et al. WebDASC: a web‐based dietary assessment software for 8–11‐year‐old Danish children. J Hum Nutr Diet 2012. e-pub ahead of print 18 May 2012 doi:10.1111/j.1365-277X.2012.01257.x.
Black A . The sensitivity and specificity of the Goldberg cut-off for EI: BMR for identifying diet reports of poor validity. Eur J Clin Nutr 2000; 54: 395–404.
Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J . Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007; 85: 660–667.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH . Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320: 1240–1245.
Cole TJ, Flegal KM, Nicholls D, Jackson AA . Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ 2007; 335: 194–197.
Dulloo AG, Jacquet J, Solinas G, Montani J-P, Schutz Y . Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int J Obes 2010; 34: 4–17.
Morris NM, Udry JR . Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolescence 1980; 9: 271–280.
Owens JA, Spirito A, McGuinn M . The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep 2000; 23: 1043–1052.
Dencker M, Thorsson O, Karlsson M, Linden C, Eiberg S, Wollmer P et al. Daily physical activity related to body fat in children aged 8-11 years. J Pediatr 2006; 149: 38–42.
Trost S, Kerr L, Ward D, Pate R . Physical activity and determinants of physical activity in obese and non-obese children. Int J Obes Relat Metab Disord 2001; 25: 822–829.
Wittmeier KD, Mollard RC, Kriellaars DJ . Objective assessment of childhood adherence to Canadian physical activity guidelines in relation to body composition. Appl Physiol Nutr Metab 2007; 32: 217–224.
Ness AR, Leary SD, Mattocks C, Blair SN, Reilly JJ, Wells J et al. Objectively measured physical activity and fat mass in a large cohort of children. PLoS med 2007; 4: e97–e105.
Ekelund U, Sardinha LB, Anderssen SA, Harro M, Franks PW, Brage S et al. Associations between objectively assessed physical activity and indicators of body fatness in 9-to 10-y-old European children: a population-based study from 4 distinct regions in Europe (the European Youth Heart Study). Am J Clin Nutr 2004; 80: 584–590.
Steele RM, van Sluijs EM, Cassidy A, Griffin SJ, Ekelund U . Targeting sedentary time or moderate-and vigorous-intensity activity: independent relations with adiposity in a population-based sample of 10-y-old British children. Am J Clin Nutr 2009; 90: 1185–1192.
Chaput JP, Lambert M, Mathieu ME, Tremblay M, O'Loughlin J, Tremblay A . Physical activity vs. sedentary time: independent associations with adiposity in children. Pediatr Obes 2012; 7: 251–258.
Fisher A, Hill C, Webber L, Purslow L, Wardle J . MVPA is associated with lower weight gain in 8–10 year old children: a prospective study with 1 year follow-up. PLoS One 2011; 6: e18576–e18581.
Moore LL, Gao D, Bradlee ML, Cupples LA, Sundarajan-Ramamurti A, Proctor MH et al. Does early physical activity predict body fat change throughout childhood? Prev Med 2003; 37: 10–17.
Stevens J, Suchindran C, Ring K, Baggett CD, Jobe JB, Story M et al. Physical activity as a predictor of body composition in American Indian children. Obes Res 2004; 12: 1974–1980.
White J, Jago R . Prospective associations between physical activity and obesity among adolescent girls: racial differences and implications for prevention. Arch Pediatr Adolesc Med 2012; 166: 522–527.
Metcalf BS, Voss LD, Hosking J, Jeffery AN, Wilkin TJ . Physical activity at the government-recommended level and obesity-related health outcomes: a longitudinal study (Early Bird 37). Arch Dis Child 2008; 93: 772–777.
Butte NF, Cai G, Cole SA, Wilson TA, Fisher JO, Zakeri IF et al. Metabolic and behavioral predictors of weight gain in Hispanic children: the Viva la Familia Study. Am J Clin Nutr 2007; 85: 1478–1485.
Stevens J, Murray DM, Baggett CD, Elder JP, Lohman TG, Lytle LA et al. Objectively assessed associations between physical activity and body composition in middle-school girls the trial of activity for adolescent girls. Am J Epidemiol 2007; 166: 1298–1305.
Trinh A, Campbell M, Ukoumunne OC, Gerner B, Wake M . Physical activity and 3-year BMI change in overweight and obese children. Pediatrics 2013; 131: e470–e477.
Ekelund U, Luan J, Sherar LB, Esliger DW, Griew P, Cooper A . Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents. JAMA 2012; 307: 704–712.
Kwon S, Janz KF, Burns TL, Levy SM . Effects of adiposity on physical activity in childhood: Iowa Bone Development Study. Med Sci Sports Exerc 2011; 43: 443–448.
Santos R, Mota J, Silva A, Baptista F, Santos DA, Sardinha LB Objectively Measured Sedentary Behavior is Associated With Body Mass Index, Independently of Physical Activity Levels, in School-Aged Portuguese Youth. Abstract http://www.actigraphcorp.com/research-database/objectively-measured-sedentary-behavior-is-associated-with-body-mass-index-independently-of-physical-activity-levels-in-school-aged-portuguese-youth/.
Mitchell JA, Pate RR, Beets MW, Nader PR . Time spent in sedentary behavior and changes in childhood BMI: a longitudinal study from ages 9 to 15 years. Int J Obes 2013; 37: 54–60.
Laurson KR, Eisenmann JC, Welk GJ, Wickel EE, Gentile DA, Walsh DA . Combined influence of physical activity and screen time recommendations on childhood overweight. J Pediatr 2008; 153: 209–214.
Boone JE, Gordon-Larsen P, Adair LS, Popkin BM . Screen time and physical activity during adolescence: longitudinal effects on obesity in young adulthood. Int J Behav Nutr Phys Act 2007; 4: 26–37.
Henderson VR . Longitudinal associations between television viewing and body mass index among white and black girls. J Adolesc Health 2007; 41: 544–550.
Ekelund U, Brage S, Besson H, Sharp S, Wareham NJ . Time spent being sedentary and weight gain in healthy adults: reverse or bidirectional causality? Am J Clin Nutr 2008; 88: 612–617.
Gupta NK, Mueller WH, Chan W, Meininger JC . Is obesity associated with poor sleep quality in adolescents? Am J Hum Biol 2002; 14: 762–768.
Nixon GM, Thompson JMD, Han DY, Becroft DM, Clark PM, Robinson E et al. Short sleep duration in middle childhood: risk factors and consequences. Sleep 2008; 31: 71–78.
Chaput JP, Lambert M, Gray-Donald K, McGrath JJ, Tremblay MS, O'Loughlin J et al. Short sleep duration is independently associated with overweight and obesity in Quebec children. Can J public health 2011; 102: 369–374.
Spruyt K, Molfese DL, Gozal D . Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics 2011; 127: e345–e352.
Hjorth MF, Chaput J-P, Michaelsen K, Astrup A, Tetens I, Sjödin A . Seasonal variation in objectively measured physical activity, sedentary time, cardio-respiratory fitness and sleep duration among 8–11 year-old Danish children: a repeated-measures study. BMC Public Health 2013; 13: 808–817.
Acknowledgements
The study is part of the OPUS project 'Optimal well-being, development and health for Danish children through a healthy New Nordic Diet' supported by a grant from the Nordea Foundation. We are very grateful to the participants and would also like to acknowledge the school staff as well as other researchers and staff in the OPUS project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
Designed research: AA, KFM, IT, S-MD and AS; coordinated data collection: MFH, S-MD and RA; analyzed and interpreted data: MFH; discussed the analysis and interpretation of the data: AS, CR and J-PC; wrote paper: MFH; had primary responsibility of the final content: AS. All authors reviewed the manuscript critically and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Hjorth, M., Chaput, JP., Ritz, C. et al. Fatness predicts decreased physical activity and increased sedentary time, but not vice versa: support from a longitudinal study in 8- to 11-year-old children. Int J Obes 38, 959–965 (2014). https://doi.org/10.1038/ijo.2013.229
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2013.229