Abstract
Background:
Obesity affects more than half a billion people worldwide, but the underlying causes remain unresolved. It has been proposed that propensity to obesity may be associated with differences between individuals in metabolic efficiency and in the energy used for homeothermy. It has also been suggested that obese-prone individuals differ in their responsiveness to circadian rhythms. We investigated both these hypotheses by measuring the core body temperature at regular and frequent intervals over a diurnal cycle, using indigestible temperature loggers in two breeds of canines known to differ in propensity to obesity, but prior to divergence in fatness.
Methods:
Greyhounds (obesity-resistant) and Labradors (obesity-prone) were fed indigestible temperature loggers. Gastrointestinal temperature was recorded at 10-min intervals for the period of transit of the logger. Diet, body condition score, activity level and environment were similar for both groups. Energy digestibility was also measured.
Results:
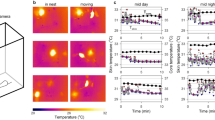
The mean core body temperature in obesity-resistant dogs (38.27 °C) was slightly higher (P<0.001) than in obesity-prone dogs (38.18 °C) and the former had a greater variation (P<0.001) in 24h circadian core temperature. There were no differences in diet digestibility.
Conclusion:
Canines differing in propensity to obesity, but prior to its onset, differed little in mean core temperature, supporting similar findings in already-obese and lean humans. Obese-prone dogs were less variable in daily core temperature fluctuations, suggestive of a degree of circadian decoupling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
WHO. www.who.int/nutrition/topics/obesity/en 2006.
Pijl H . Obesity: evolution of a symptom of affluence. J Med 2011; 69: 159–166.
Landsberg L . Core temperature: a forgotten variable in energy expenditure and obesity? Obesity Rev 2012; 13 (Suppl 2): 97–104.
Neel JV . Diabetes mellitus: a ‘thrifty’ genotype rendered detrimental by ‘progress’? Am J Hum Genet 1962; 14: 353–362.
Landsberg L, Young JB, Leonard WR, Linsenmeier RA, Turek FW . Do the obese have lower body temperatures? A new look at a forgotten variable in energy balance. Trans Am Clin Climatol Assoc 2009; 120: 287–295.
Landsberg L, Young JB . Autonomic regulation of thermogenesis. In Giradier L, Stock MJ, editors. Mammalian Thermogenesis 1st edn. Chapman and Hall: London, UK, 1983. pp 99–140.
Girardier L, Stock MJ . Mammalian thermogenesis: an introduction. In Giradier L, Stock MJ, editors. Mammalian Thermogenesis 1st edn. Chapman and Hall: London, UK, 1983. pp 1–8.
Westerterp KR, Speakman JR . Physical activity energy expenditure has not declined since the 1980s and matches energy expenditure of wild mammals. Int J Obes 2008; 32: 1256–1263.
Dubois EF . The basal metabolism in fever. JAMA 1921; 77: 352–355.
Davis TRA, Mayer J . Imperfect homeothermia in the hereditary obese-hyperglycemic syndrome of mice. Am J Physiol 1954; 177: 222–226.
Hammel HT, Elsner RW, LeMessurier DH, Andersen HT, Milan FA . Thermal and metabolic responses of the Australian aborigine exposed to moderate cold in summer. J Appl Physiol 1959; 14: 605–615.
Rising R, Keys A, Ravussin E, Bogardus C . Concomitant interindividual variation in body temperature and metabolic rate. Am J Physiol 1992; 263: E730–E734.
Adam K . Human body temperature is inversely correlated with body mass. Eur J Appl Physiol 1989; 58: 471–475.
Kim H, Richardson C, Roberts J, Gren L, Lyon JL . Cold hands, warm heart. Lancet 1998; 351: 1492.
Piccione G, Giudice E, Fazio F, Refinetti R . Association between obesity and reduced body temperature in dogs. Int J Obesity 2011; 35: 1011–1018.
Rising R, Fontvieille AM, Larson DE, Spraul M, Bogardus C, Ravussin E . Racial difference in body core temperature between Pima Indian and Caucasian men. Int J Obes 1995; 19: 1–5.
Ericksson H, Svardsudd K, Larsson B, Welin L, Ohlson LO, Wilhelmsen L . Body temperature in general population samples. The study of men born in 1913 and 1923. Acta Med Scand 1985; 217: 347–352.
Hoffman ME, Rodriguez SM, Zeiss DM, Wachsberg KN, Kushnerr RF, Landsberg L, Linsenmeier RA . 24-hour core body temperature in obese and lean men and women. Obesity (Silver Spring) 2012; 20: 1585–1590.
Marcheva B, Ramsey KM, Affinati A, Bass JJ . Clock genes and metabolic disease. Appl Physiol 2009; 107: 1638–1646.
Wyse CA . Does human evolution in different latitudes influence susceptibility to obesity via the circadian pacemaker? Bioessays 2012; 34: 921–924.
Corbalan-Tutau MD, Madrid JA, Ordovas JM, Smith CE, Nicolas F, Garaulet M . Differences in daily rhythms of wrist temperature between obese and normal weight women: associations with metabolic syndrome features. Chronobiol Int 2011; 28: 425–443.
Bland IM, Guthrie-Jones A, Taylor RD, Jill J . Dog obesity: owner attitudes and behaviour. Prev Vet Med 2008; 92: 333–340.
Lund EM, Armstrong PJ, Kirk CA, Klauser JS . Prevalence and risk factors for obesity in adult dogs from private practices. Int J Appl Res Vet Med 2006; 4: 177–186.
McGreevy PD, Thomson PC, Pride C, Fawcett A, Grassi T, Jones B . Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved. Vet Rec 2012; 156: 695–702.
Edney ATB, Smith PM . Study of obesity in dogs visiting veterinary practices in the United Kingdom. Vet Rec 1986; 118: 391–396.
Kronfeld DS, Donoghue S, Glickman LT . Body condition and energy intake of dogs in a referral teaching hospital. J Nutr 1991; 121 (Suppl S): 157–158.
Zoran DL . Obesity in dogs and cats: a metabolic and endocrine disorder. Vet Clin Small Anim 2010; 40: 221–239.
Diez M, Nguyen P . The epidemiology of canine and feline obesity. Waltham Focus 2006; 16: 2–8.
Wyse CA, Selman C, Page MM, Coogan AN, Haelrigg DG . Circadian desynchrony and metabolic dysfunction; did light pollution make us fat? Med Hypoth 2011; 77: 1139–1144.
Saad RJ, Hasler WL . A technical review and clinical assessment of the wireless motility capsule. Gastroenterol Hepatol 2011; 7: 795–804.
Kreider MB, Buskirk ER, Bass DE . Oxygen consumption and body temperatures during the night. J Appl Physiol 1958; 12: 361–366.
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science 2005; 308: 1043–1045.
Windham BG, Fumagalli S, Ble A, Sollers JJ, Thayer JF, Naijar SS, Griswold ME, Ferrucci L . The relationship between heart rate variability and adiposity differs for central and overall adiposity. J Obes 2012; 2012: 149516.
Acknowledgements
We wish to thank the staff at the Royal Society for the Blind Pty Ltd South Australia for providing access to the Labrador Retriever guide dogs. In particular, we thank Dr Chris Muldoon, Ms Celeste Osmond, Ms Daisy Piccoli and the foster-carers of the guide dogs. Dr Jane McNicholl is also gratefully acknowledged for assistance in accessing Greyhounds for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hynd, P., Czerwinski, V. & McWhorter, T. Is propensity to obesity associated with the diurnal pattern of core body temperature?. Int J Obes 38, 231–235 (2014). https://doi.org/10.1038/ijo.2013.110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2013.110