Abstract
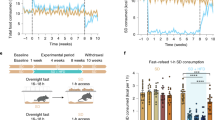
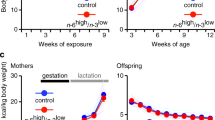
We have previously demonstrated that exposure to high fat (HF) during early development alters the presynaptic regulation of mesolimbic dopamine (DA), and increases incentive motivation for HF food rewards. The goal of the present experiments was to examine the long-term consequences of early exposure to HF on anticipatory and consumatory nucleus accumbens (NAc) DA responses to HF food rewards. Mothers were maintained on a HF (30% fat) or control diet (CD; 5% fat) from gestation day 13 to postnatal day 22 when offspring from both diet groups were weaned and maintained on the CD until adulthood. In vivo NAc DA responses to food anticipation and consumption were measured in a Pavlovian conditioning paradigm using voltammetry in freely moving rats. HF-exposed offspring displayed reduced NAc DA responses to a tone previously paired with the delivery of HF food rewards. In an unconditioned protocol, consumatory NAc DA responses could be isolated, and were similar in HF and control offspring. These data demonstrate that exposure to HF through maternal diet during early development might program behavioral and functional responses associated with mesolimbic DA neurotransmission, thus leading to an increased HF feeding and obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Levin BE . Metabolic imprinting: critical impact of the perinatal environment on the regulation of energy homeostasis. Philos Trans Royal Soc Lond B Biol Sci 2006; 361: 1107–1121.
Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM . Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 2008; 117: 924–935.
Frank GKW, Reynolds JR, Shott ME, Jappe L, Yang TT, Tregellas JR et al. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology 2012; 37: 2031–2046.
Green E, Jacobson A, Haase L, Murphy C . Reduced nucleus accumbens and caudate nucleus activation to a pleasant taste is associated with obesity in older adults. Brain Res 2011; 1386: 109–117.
Davis JF, Tracy AL, Shurdak JD, Tschop MT, Lipton JW, Clegg DJ et al. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci 2008; 122: 1257–1263.
Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN . Deficits in mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 2009; 159: 1193–1199.
Li Y, South T, Han M, Chen J, Wang R, Huang XF . High-fat diet decreases tyrosine hydroxylase mRNA expression irrespective of obesity susceptibility in mice. Brain Res 2009; 1268: 181–189.
Sharma S, Fulton S . Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obes 2012. e-pub ahead of print 17 April 2012; doi.10.1038/ijo.2012.48.
Antonopoulos J, Dori I, Dinopoulos A, Chiotelli M, Parnavelas JG . Postnatal development of the dopaminergic system of the striatum in the rat. Neuroscience 2002; 110: 245–256.
Teegarden SL, Scott AN, Bale TL . Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience 2009; 162: 924–932.
Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM . Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 2010; 151: 4756–4764.
Naef L, Moquin L, Dal Bo G, Giros B, Gratton A, Walker CD . Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience 2010; 176: 225–236.
Naef L, Srivastava L, Gratton A, Hendrickson H, Owens SM, Walker CD . Maternal high fat diet during the perinatal period alters mesocorticolimbic dopamine in the adult rat offspring: reduction on the behavioral responses to repeated amphetamine administration. Psychopharmacology 2008; 197: 83–94.
Richardson NR, Gratton A . Changes in nucleus accumbens dopamine transmission associated with fixed- and variable-time schedule-induced feeding. Eur J Neurosci 2008; 27: 2714–2723.
Paxinos G, Watson C . The Rat Brain in Stereotaxic Coordinates. 4th edn. Academic Press: Boston, 1998.
Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG et al. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J 2008; 22: 2740–2746.
Berthoud HR, Lenard NR, Shin AC . Food reward, hyperphagia, and obesity. Am J Physiol Regul Integr Comp Physiol 2011; 300: R1266–R1277.
Baldo BA, Kelley AE . Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 2007; 191: 439–459.
Acknowledgements
This research was supported by a grant from CIHR (no. 84299) to C-DW and AG. LN is a recipient of a CIHR Canada Graduate Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Naef, L., Moquin, L., Gratton, A. et al. Reduced anticipatory dopamine responses to food in rats exposed to high fat during early development. Int J Obes 37, 885–888 (2013). https://doi.org/10.1038/ijo.2012.153
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2012.153