Abstract
Context:
The association between low socioeconomic status (SES) and childhood obesity foreshadows lifelong inequalities in health. Insight into the causal mechanisms linking childhood adversity to long-term health could be provided by discovering when the negative SES gradient in weight emerges and what early life experiences are associated with it.
Objective:
SES differences in infant weight gain in the first 3 months of life were examined, and contributions of parental body mass index, maternal smoking and feeding method to this association were assessed.
Design:
Observational study using longitudinal weight data from 2402 families taking part in the Gemini Study; a twin birth cohort recruited from all twin births between March and December 2007 in England and Wales.
Outcome measures:
Infant weights at birth and 3 months converted to standard deviation scores (SDS), change in weight SDS and rapid growth. SES was indexed by occupation and maternal education.
Results:
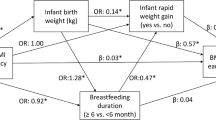
There were no SES differences in birth weight, but lower SES was associated with higher 3-month weight, greater change in weight and a higher prevalence of rapid growth (all P<0.01), with graded associations across levels of SES. Including parental overweight or smoking in pregnancy in the regression model did not affect the association between SES and weight gain, but including feeding method attenuated the SES effect on weight gain by at least 62% and rendered it nonsignificant.
Conclusion:
The foundations for lifelong socioeconomic inequalities in obesity risk may be laid in early infancy, with infant-feeding practices having a part in the diverging weight trajectories.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Simmons R . Perinatal programming of obesity. Semin Perinatol 2008; 32: 371–374.
Dennison BA, Edmunds LS, Stratton HH, Pruzek RM . Rapid infant weight gain predicts childhood overweight. Obesity (Silver Spring) 2006; 14: 491–499.
Ong KK, Loos RJ . Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr 2006; 95: 904–908.
Herman KM, Craig CL, Gauvin L, Katzmarzyk PT . Tracking of obesity and physical activity from childhood to adulthood: the Physical Activity Longitudinal Study. Int J Pediatr Obes 2009; 4: 281–288.
Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ . Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics 2004; 113: 475–482.
Hannon TS, Rao G, Arslanian SA . Childhood obesity and type 2 diabetes mellitus. Pediatrics 2005; 116: 473–480.
Gunnell DJ, Frankel SJ, Nanchahal K, Peters TJ, Davey SG . Childhood obesity and adult cardiovascular mortality: a 57-y follow-up study based on the Boyd Orr cohort. Am J Clin Nutr 1998; 67: 1111–1118.
Biro FM, Wien M . Childhood obesity and adult morbidities. Am J Clin Nutr 2010; 91 (Suppl): 1499S–1505S.
Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A . Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA 2009; 301: 2234–2242.
Dubois L, Girard M . Determinants of birthweight inequalities: population-based study. Pediatr Int 2006; 48: 470–478.
Jansen PW, Tiemeier H, Looman CW, Jaddoe VW, Hofman A, Moll HA et al. Explaining educational inequalities in birthweight: the Generation R Study. Paediatr Perinat Epidemiol 2009; 23: 216–228.
Kramer MS, Seguin L, Lydon J, Goulet L . Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinat Epidemiol 2000; 14: 194–210.
Semmler C, Ashcroft J, van Jaarsveld CH, Carnell S, Wardle J . Development of overweight in children in relation to parental weight and socioeconomic status. Obesity (Silver Spring) 2009; 17: 814–820.
Langnase K, Mast M, Muller MJ . Social class differences in overweight of prepubertal children in northwest Germany. Int J Obes Relat Metab Disord 2002; 26: 566–572.
Stamatakis E, Wardle J, Cole TJ . Childhood obesity and overweight prevalence trends in England: evidence for growing socioeconomic disparities. Int J Obes (Lond) 2010; 34: 41–47.
Dubois L, Girard M . Early determinants of overweight at 4.5 years in a population-based longitudinal study. Int J Obes (Lond) 2006; 30: 610–617.
McLaren L . Socioeconomic status and obesity. Epidemiol Rev 2007; 29: 29–48.
Whitaker KL, Jarvis MJ, Beeken RJ, Boniface D, Wardle J . Comparing maternal and paternal intergenerational transmission of obesity risk in a large population-based sample. Am J Clin Nutr 2010; 91: 1560–1567.
Laaksonen M, Rahkonen O, Karvonen S, Lahelma E . Socioeconomic status and smoking: analysing inequalities with multiple indicators. Eur J Public Health 2005; 15: 262–269.
Morgen CS, Bjork C, Andersen PK, Mortensen LH, Nybo Andersen AM . Socioeconomic position and the risk of preterm birth--a study within the Danish National Birth Cohort. Int J Epidemiol 2008; 37: 1109–1120.
Lanting CI, Buitendijk SE, Crone MR, Segaar D, Bennebroek Gravenhorst J, Wouwe JP . Clustering of socioeconomic, behavioural, and neonatal risk factors for infant health in pregnant smoker. Plos One 2009; 4: e8363.
Conter V, Cortinovis I, Rogari P, Riva L . Weight growth in infants born to mothers who smoked during pregnancy. BMJ 1995; 310: 768–771.
van Rossem L, Oenema A, Steegers EA, Moll HA, Jaddoe VW, Hofman A et al. Are starting and continuing breastfeeding related to educational background? The generation R study. Pediatrics 2009; 123: e1017–e1027.
Kramer MS, Guo T, Platt RW, Vanilovich I, Sevkovskaya Z, Dzikovich I et al. Feeding effects on growth during infancy. J Pediatr 2004; 145: 600–605.
Rzehak P, Sausenthaler S, Koletzko S, Bauer CP, Schaaf B, von Berg A et al. Period-specific growth, overweight and modification by breastfeeding in the GINI and LISA birth cohorts up to age 6 years. Eur J Epidemiol 2009; 24: 449–467.
van Jaarsveld CH, Johnson L, Llewellyn C, Wardle J . Gemini: a UK twin birth cohort with a focus on early childhood weight trajectories, appetite and the family environment. Twin Res Hum Genet 2010; 13: 72–78.
Office for National Statistics. Review of the Registrar General on Births and Patterns of Family Building in England and Wales. National Statistics: Newport, UK, 2006.
Office for National Statistics. National Statistics Socio-economic Classification: User Manual. Office for National Statistics: Newport, UK, 2005.
Jones R, Elias P . CASCOT: Computer Assisted Coding Tool. http://www2.warwick.ac.uk/fac/soc/ier/publications/software/cascot/.
Office for National Statistics. Standard Occupational Classification 2000 (SOC 2000) Vol. 1. Office for National Statistics: Newport, UK, 2000.
Office for National Statistics. Standard Occupational Classification 2000 (SOC 2000) Vol. 2. Office for National Statistics: Newport, UK, 2000.
Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA . Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child 1995; 73: 17–24.
Cole TJ . Software for LMS method: LMSGrowth PC. http://homepage.mac.com/tjcole/FileSharing1.html.
Pollack H, Lantz PM, Frohna JG . Maternal smoking and adverse birth outcomes among singletons and twins. Am J Public Health 2000; 90: 395–400.
Baker JL, Michaelsen KF, Rasmussen KM, Sorensen TI . Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr 2004; 80: 1579–1588.
Bartok CJ, Ventura AK . Mechanisms underlying the association between breastfeeding and obesity. Int J Pediatr Obes 2009; 4: 196–204.
Taveras EM, Scanlon KS, Birch L, Rifas-Shiman SL, Rich-Edwards JW, Gillman MW . Association of breastfeeding with maternal control of infant feeding at age 1 year. Pediatrics 2004; 114: e577–e583.
Taveras EM, Rifas-Shiman SL, Scanlon KS, Grummer-Strawn LM, Sherry B, Gillman MW . To what extent is the protective effect of breastfeeding on future overweight explained by decreased maternal feeding restriction? Pediatrics 2006; 118: 2341–2348.
Szyf M . The early life environment and the epigenome. Biochim Biophys Acta 2009; 1790: 878–885.
Wilson RS . Twin growth: initial deficit, recovery, and trends in concordance from birth to nine years. Ann Hum Biol 1979; 6: 205–220.
Teranishi H, Nakagawa H, Marmot M . Social class difference in catch up growth in a national British cohort. Arch Dis Child 2001; 84: 218–221.
Li L, Manor O, Power C . Early environment and child-to-adult growth trajectories in the 1958 British birth cohort. Am J Clin Nutr 2004; 80: 185–192.
Office for National Statistics. Census 2001. http://www.statistics.gov.uk/census2001/census2001.asp.
Griffiths LJ, Smeeth L, Hawkins SS, Cole TJ, Dezateux C . Effects of infant feeding practice on weight gain from birth to 3 years. Arch Dis Child 2009; 94: 577–582.
Arenz S, Ruckerl R, Koletzko B, von KR . Breast-feeding and childhood obesity—a systematic review. Int J Obes Relat Metab Disord 2004; 28: 1247–1256.
Kramer MS, Matush L, Vanilovich I, Platt RW, Bogdanovich N, Sevkovskaya Z et al. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. Am J Clin Nutr 2007; 86: 1717–1721.
Carnell S, Wardle J . Appetite and adiposity in children: evidence for a behavioral susceptibility theory of obesity. Am J Clin Nutr 2008; 88: 22–29.
Carnell S, Haworth CM, Plomin R, Wardle J . Genetic influence on appetite in children. Int J Obes (Lond) 2008; 32: 1468–1473.
Acknowledgements
The Gemini Study is funded by a grant from the Cancer Research UK to JW (C1418/A7974). The funding organizations had no role in the design and conduct of the study; collection, management, analysis and interpretation of data, and preparation, review or approval of the manuscript. We thank the Gemini families who are participating in the study and the Office of National Statistics for their help in recruiting them.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wijlaars, L., Johnson, L., van Jaarsveld, C. et al. Socioeconomic status and weight gain in early infancy. Int J Obes 35, 963–970 (2011). https://doi.org/10.1038/ijo.2011.88
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.88