Abstract
Objective:
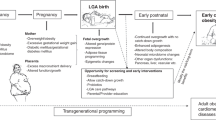
To investigate whether delivery mode (vaginal versus by caesarean section), maternal pre-pregnancy body mass index (BMI) and early exposure to antibiotics (<6 months of age) influence child's risk of overweight at age 7 years, hence supporting the hypotheses that environmental factors influencing the establishment and diversity of the gut microbiota are associated with later risk of overweight.
Design:
Longitudinal, prospective study with measure of exposures in infancy and follow-up at age 7 years.
Methods:
A total of 28 354 mother–child dyads from the Danish National Birth Cohort, with information on maternal pre-pregnancy BMI, delivery mode and antibiotic administration in infancy, were assessed. Logistic regression analyses were performed with childhood height and weight at the 7-year follow-up as outcome measures.
Results:
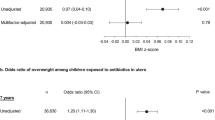
Delivery mode was not significantly associated with childhood overweight (odds ratio (OR):1.18, 95% confidence interval (CI): 0.95–1.47). Antibiotics during the first 6 months of life led to increased risk of overweight among children of normal weight mothers (OR: 1.54, 95% CI: 1.09–2.17) and a decreased risk of overweight among children of overweight mothers (OR: 0.54, 95% CI: 0.30–0.98). The same tendency was observed among children of obese mothers (OR: 0.85, 95% CI: 0.41–1.76).
Conclusion:
The present cohort study revealed that a combination of early exposures, including delivery mode, maternal pre-pregnancy BMI and antibiotics in infancy, influences the risk of overweight in later childhood. This effect may potentially be explained by an impact on establishment and diversity of the microbiota.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schousboe K, Visscher PM, Erbas B, Kyvik KO, Hopper JL, Henriksen JE et al. Twin study of genetic and environmental influences on adult body size, shape, and composition. Int J Obes Relat Metab Disord 2004; 28: 39–48.
Silventoinen K, Rokholm B, Kaprio J, Sørensen TIA . The genetic and environmental influences on childhood obesity: a systematic review of twin and adoption studies. Int J Obes (Lond) 2010; 34: 29–40.
Whitaker KL, Jarvis MJ, Beeken RJ, Boniface D, Wardle J . Comparing maternal and paternal intergenerational transmission of obesity risk in a large population-based sample. Am J Clin Nutr 2010; 91: 1560–1567.
Whitaker RC . Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics 2004; 114: e29–e36.
Cani PD, Delzenne NM . The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des 2009; 15: 1546–1558.
Bedford Russell AR, Murch SH . Could peripartum antibiotics have delayed health consequences for the infant? BJOG 2006; 113: 758–765.
Biasucci G, Benenati B, Morelli L, Bessi E, Boehm G . Cesarean delivery may affect the early biodiversity of intestinal bacteria. J Nutr 2008; 138: 1796S–1800S.
Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C . Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev 2010; 86 (Suppl 1): 13–15.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI . Microbial ecology: human gut microbes associated with obesity. Nature 2006; 444: 1022–1023.
Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006; 118: 511–521.
Phillips ML . Gut reaction: environmental effects on the human microbiota. Environ Health Perspect 2009; 117: A198–A205.
Reinhardt C, Reigstad CS, Backhed F . Intestinal microbiota during infancy and its implications for obesity. J Pediatr Gastroenterol Nutr 2009; 48: 249–256.
Salminen S, Gibson GR, McCartney AL, Isolauri E . Influence of mode of delivery on gut microbiota composition in seven year old children. Gut 2004; 53: 1388–1389.
Salminen S, Gueimonde M . Gut microbiota in infants between 6 and 24 months of age. Nestle Nutr Workshop Ser Pediatr Program 2005; 56: 43–51.
Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE et al. A core gut microbiome in obese and lean twins. Nature 2009; 457: 480–484.
Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 2004; 101: 15718–15723.
Backhed F . Changes in intestinal microflora in obesity: cause or consequence? J Pediatr Gastroenterol Nutr 2009; 48 (Suppl 2): S56–S57.
Huurre A, Kalliomaki M, Rautava S, Rinne M, Salminen S, Isolauri E . Mode of delivery—effects on gut microbiota and humoral immunity. Neonatology 2008; 93: 236–240.
Penders J, Stobberingh EE, van den Brandt PA, Thijs C . The role of the intestinal microbiota in the development of atopic disorders. Allergy 2007; 62: 1223–1236.
Lobstein T, Baur L, Uauy R . Obesity in children and young people: a crisis in public health. Obes Rev 2004; 5 (Suppl 1): 4–104.
Oken E, Levitan EB, Gillman MW . Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond) 2008; 32: 201–210.
Arenz S, Von KR . Protective effect of breast-feeding against obesity in childhood: can a meta-analysis of published observational studies help to validate the hypothesis? Adv Exp Med Biol 2009; 639: 145–152.
Olsen J, Melbye M, Olsen SF, Sørensen TIA, Aaby P, Andersen AM et al. The Danish National Birth Cohort—its background, structure and aim. Scand J Public Health 2001; 29: 300–307.
The Danish National Birth Cohort. www.dnbc.dk 2010.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH . Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320: 1240–1243.
Cole TJ, Flegal KM, Nicholls D, Jackson AA . Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ 2007; 335: 194.
Collado MC, Isolauri E, Laitinen K, Salminen S . Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr 2008; 88: 894–899.
Kalliomaki M, Collado MC, Salminen S, Isolauri E . Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr 2008; 87: 534–538.
Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut 2007; 56: 661–667.
Ternak G . Antibiotics may act as growth/obesity promoters in humans as an inadvertent result of antibiotic pollution? Med Hypotheses 2005; 64: 14–16.
Isolauri E, Kalliomaki M, Rautava S, Salminen S, Laitinen K . Obesity—extending the hygiene hypothesis. Nestle Nutr Workshop Ser Pediatr Program 2009; 64: 75–85.
Hesketh K, Carlin J, Wake M, Crawford D . Predictors of body mass index change in Australian primary school children. Int J Pediatr Obes 2009; 4: 45–53.
Nohr EA, Frydenberg M, Henriksen TB, Olsen J . Does low participation in cohort studies induce bias? Epidemiology 2006; 17: 413–418.
Craig BM, Adams AK . Accuracy of body mass index categories based on self-reported height and weight among women in the United States. Matern Child Health J 2009; 13: 489–496.
Toda T, Saito N, Ikarashi N, Ito K, Yamamoto M, Ishige A et al. Intestinal flora induces the expression of Cyp3a in the mouse liver. Xenobiotica 2009; 39: 323–334.
Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr 2009; 90: 1236–1243.
Kalliomaki M, Salminen S, Poussa T, Isolauri E . Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol 2007; 119: 1019–1021.
Acknowledgements
The study was financed through a Female Research Leader grant (no. 09-066323) from the Danish Council of Independent Research to Dr Tine Jess. Additional support for the DNBC (http://www.dnbc.dk) was obtained from the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Augustinus Foundation and the Health Foundation. The 7-year follow-up study within the DNBC has received financial support from the Lundbeck Foundation (195/04) and the Danish Medical Research Council (SSVF 0646). We acknowledge all the families who are represented in the cohort for their thorough contribution in completing questionnaires. This work was also carried out as a part of the research programme of the Danish Obesity Research Centre (DanORC, see http://www.danorc.dk), granted by the Danish Council for Strategic Research (grant no. 2101-06-0005), and as part of the TORNADO collaboration (http://www.fp7tornado.eu).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ajslev, T., Andersen, C., Gamborg, M. et al. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes 35, 522–529 (2011). https://doi.org/10.1038/ijo.2011.27
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.27
Keywords
This article is cited by
-
Evaluating the association between caesarean delivery and weight status in early childhood in a Japanese birth cohort study
Scientific Reports (2023)
-
Early-life antibiotic exposure increases the risk of childhood overweight and obesity in relation to dysbiosis of gut microbiota: a birth cohort study
Annals of Clinical Microbiology and Antimicrobials (2022)
-
Antibiotics prior to age 2 years have limited association with preschool growth trajectory
International Journal of Obesity (2022)
-
Antibiotic exposure and growth patterns in preterm, very low birth weight infants
Maternal Health, Neonatology and Perinatology (2021)
-
Preliminary evidence for an influence of exposure to polycyclic aromatic hydrocarbons on the composition of the gut microbiota and neurodevelopment in three-year-old healthy children
BMC Pediatrics (2021)