Abstract
Objective:
The aim of this functional magnetic resonance imaging (fMRI) study was to investigate reward-related brain activity in satiated overweight and healthy-weight participants in response to high-calorie palatable food pictures, when viewing the pictures without prior instructions (called unbiased viewing) versus imagining the taste of the shown pictures (called taste imagination). We predicted that neural activation in brain reward regions would be greater in overweight participants than in healthy-weight ones and that this difference between groups would be strongest during unbiased viewing.
Method:
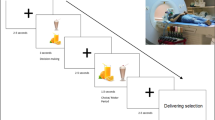
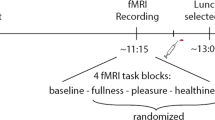
Neural activation was measured using fMRI in 14 overweight (mean body mass index (BMI): 29.8 kg m−2) and 15 healthy-weight (mean BMI: 21.1 kg m−2) participants who were satiated, in response to palatable and unpalatable high-calorie and low-calorie food pictures, presented in an event-related design during two conditions: unbiased viewing (no prior instructions) versus taste imagination.
Results:
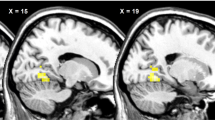
A group × condition interaction was found in 14 brain regions involved in food reward processing during the presentation of high-calorie palatable food stimuli. During the taste imagination condition, neural activation in these regions was greater in the overweight participants than in the healthy-weight ones. Contrary to our expectations, the opposite pattern was observed during unbiased viewing: activation in reward regions in the overweight participants was reduced compared with the healthy-weight ones. In all brain reward regions except for the left amygdala, the group × condition interaction was specific to high-calorie palatable food stimuli.
Conclusion:
Greater reward activity in the overweight participants compared with the control group when imagining taste may represent an increased reward response induced by high-calorie palatable food. During unbiased viewing, reduced reward activation in the overweight participants compared with those with a healthy weight may reflect avoidance of high-calorie palatable food stimuli. Taken together, this pattern of activation may reflect ambivalence in the overweight group between desire for (in the taste imagination condition) and avoidance of (in the unbiased viewing condition) high-calorie palatable food stimuli.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Epstein LH, Leddy JJ, Temple JL, Faith MS . Food reinforcement and eating: a multilevel analysis. Psychol Bull 2007; 133: 884–906.
Hill JO, Catenacci V, Wyatt HR . Obesity: overview of an epidemic. Psychiatr Clin North Am 2005; 28: 1–23 vii.
Schoeller DA . The energy balance equation: looking back and looking forward are two very different views. Nutr Rev 2009; 67: 249–254.
Berghöfer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SN . Obesity prevalence from a European perspective: a systematic review. BMC Public Health 2008; 8: 200.
Kelly T, Yang W, Chen C-S, Reynolds K, He J . Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008; 32: 1431–1437.
Branca F, Nikogosian H, Lobstein T (eds). The Challenge of Obesity in the WHO European Region and the Strategies for Response: Summary. World Health Organization: Copenhagen, 2007.
Drewnowski A . The real contribution of added sugars and fats to obesity. Epidemiol Rev 2007; 29: 160–171.
Rolls BJ . The role of energy density in the overconsumption of fat. J Nutr 2000; 130 (2S Suppl): 268S–271S.
McCrory MA, Fuss PJ, McCallum JE, Yao M, Vinken AG, Hays NP et al. Dietary variety within food groups: association with energy intake and body fatness in men and women. Am J Clin Nutr 1999; 69: 440–447.
Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ . Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci 2007; 121: 877–886.
Giesen JCAH, Havermans R, Douven A, Tekelenburg M, Jansen A . Will work for snack food: the association of BMI and snack reinforcement. Obesity (Silver Spring) 2010; 18: 966–970.
Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C . From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite 2007; 48: 12–19.
Franken IHA, Muris P . Individual differences in reward sensitivity are related to food craving and relative body weight in healthy women. Appetite 2005; 45: 198–201.
van der Laan LN, de Ridder DTD, Viergever MA, Smeets PAM . The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. NeuroImage 2011; 55: 296–303.
Kringelbach ML . The hedonic brain: a functional neuroanatomy of human pleasure. In: Kringelbach ML, Berridge KC (eds). Pleasures of the Brain. Oxford University Press: New York, 2009, pp 202–221.
Berthoud H-R, Lenard NR, Shin AC . Food reward, hyperphagia, and obesity. Am J Physiol Regul Integr Comp Physiol 2011; 300: R1266–R1277.
Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE . Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage 2008; 41: 636–647.
Rothemund Y, Preuschhof C, Bohner G, Bauknecht H-C, Klingebiel R, Flor H et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage 2007; 37: 410–421.
Wallner-Liebmann S, Koschutnig K, Reishofer G, Sorantin E, Blaschitz B, Kruschitz R et al. Insulin and hippocampus activation in response to images of high-calorie food in normal weight and obese adolescents. Obesity (Silver Spring) 2010; 18: 1552–1557.
Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM . Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 2008; 117: 924–935.
Stoeckel LE, Kim J, Weller RE, Cox JE, Cook EW, Horwitz B . Effective connectivity of a reward network in obese women. Brain Res Bull 2009; 79: 388–395.
Batterink L, Yokum S, Stice E . Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. NeuroImage 2010; 52: 1696–1703.
Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A . Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res 2009; 198: 149–158.
Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC . Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci 2003; 23: 9632–9638.
Gottfried JA, O’Doherty JP, Dolan RJ . Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 2003; 301: 1104–1107.
Bender G, Veldhuizen MG, Meltzer JA, Gitelman DR, Small DM . Neural correlates of evaluative compared with passive tasting. Eur J Neurosci 2009; 30: 327–338.
Cornier M-A, Rojas DC, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR . The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS One 2009; 4: e6310.
Fisher JO, Birch LL . Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. Am J Clin Nutr 2002; 76: 226–231.
Zheng H, Lenard NR, Shin AC, Berthoud H-R . Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 2009; 33 (Suppl 2): S8–S13.
Dreher J-C, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF . Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A 2007; 104: 2465–2470.
Bryant M, Truesdale KP, Dye L . Modest changes in dietary intake across the menstrual cycle: implications for food intake research. Br J Nutr 2006; 96: 888–894.
Guillebaud J . The mechanism of the pill. In: Gunn ADG (ed). Oral Contraception in Perspective. Thirty Years of Clinical Experience with the Pill. The Parthenon Publishing Group: New Jersey, USA, 1987, pp 75–84.
Friedman MI, Ulrich P, Mattes RD . A figurative measure of subjective hunger sensations. Appetite 1999; 32: 395–404.
Stubbs RJ, Hughes DA, Johnstone AM, Rowley E, Reid C, Elia M et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr 2000; 84: 405–415.
Williamson DA, Martin CK, York-Crowe E, Anton SD, Redman LM, Han H et al. Measurement of dietary restraint: validity tests of four questionnaires. Appetite 2007; 48: 183–192.
van Strien T, Herman CP, Engels RCME, Larsen JK, van Leeuwe JFJ . Construct validation of the Restraint scale in normal-weight and overweight females. Appetite 2007; 49: 109–121.
Herman CP, Polivy J . Restrained eating. In: Stunkard AJ (ed). Obesity. Saunders: Philadelphia, 1980, pp 208–225.
Scagliusi FB, Polacow VO, Cordás TA, Coelho D, Alvarenga M, Philippi ST et al. Test-retest reliability and discriminant validity of the Restraint scale translated into Portuguese. Eat Behav 2005; 6: 85–93.
Watson D, Clark LA, Tellegen A . Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988; 54: 1063–1070.
Killgore WDS, Yurgelun-Todd DA . Affect modulates appetite-related brain activity to images of food. Int J Eat Disord 2006; 39: 357–363.
Serences JT . A comparison of methods for characterizing the event-related BOLD timeseries in rapid fMRI. NeuroImage 2004; 21: 1690–1700.
Deichmann R, Gottfried JA, Hutton C, Turner R . Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage 2003; 19 (2 Pt 1): 430–441.
Weiskopf N, Hutton C, Josephs O, Turner R, Deichmann R . Optimized EPI for fMRI studies of the orbitofrontal cortex: compensation of susceptibility-induced gradients in the readout direction. MAGMA 2007; 20: 39–49.
Mugler JP, Brookeman JR . Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med 1990; 15: 152–157.
Deichmann R, Good CD, Josephs O, Ashburner J, Turner R . Optimization of 3-D MP-RAGE sequences for structural brain imaging. NeuroImage 2000; 12: 112–127.
Talairach J, Tournoux P . Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System - an Approach to Cerebral Imaging. Thieme Medical Publishers: New York, 1988.
Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R . Event-related fMRI: characterizing differential responses. NeuroImage 1998; 7: 30–40.
Goebel R, Esposito F, Formisano E . Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp 2006y; 27: 392–401.
Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC . Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995; 33: 636–647.
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 2000; 10: 120–131.
Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T . Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron 2003; 39: 701–711.
Veenstra EM, de Jong PJ, Koster EHW, Roefs A . Attentional avoidance of high-fat food in unsuccessful dieters. J Behav Ther Exp Psychiatry 2010; 41: 282–288.
Werthmann J, Roefs A, Nederkoorn C, Mogg K, Bradley BP, Jansen A . Can(not) take my eyes off it: attention bias for food in overweight participants. Health Psychol 2011; 30: 561–569.
Bond DS, Raynor HA, Vithiananthan S, Sax HC, Pohl D, Roye GD et al. Differences in salivary habituation to a taste stimulus in bariatric surgery candidates and normal-weight controls. Obes Surg 2009; 19: 873–878.
Epstein LH, Robinson JL, Temple JL, Roemmich JN, Marusewski A, Nadbrzuch R . Sensitization and habituation of motivated behavior in overweight and non-overweight children. Learn Motiv 2008; 39: 243–255.
McCaffery JM, Haley AP, Sweet LH, Phelan S, Raynor HA, Del Parigi A et al. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am J Clin Nutr 2009; 90: 928–934.
Haase L, Green E, Murphy C . Males and females show differential brain activation to taste when hungry and sated in gustatory and reward areas. Appetite 2011; 57: 421–434.
Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC . Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res 2006; 169: 111–119.
Hill AJ . The psychology of food craving. Proc Nutr Soc 2007; 66: 277–285.
Rogers PJ, Smit HJ . Food craving and food ‘addiction’: a critical review of the evidence from a biopsychosocial perspective. Pharmacol Biochem Behav 2000; 66: 3–14.
Acknowledgements
We thank Charlie Bonnemayer of Maastricht University for his help in programming the stimulus protocol in E-Prime, Dr Armin Heinecke of Brain Innovation B.V. for his help with the data analysis and Dr Samantha Brooks for proofreading of the manuscript. This study was financed by the Netherlands Research Organization (NWO) grant number 400.06.148, awarded to Anita Jansen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Frankort, A., Roefs, A., Siep, N. et al. Reward activity in satiated overweight women is decreased during unbiased viewing but increased when imagining taste: an event-related fMRI study. Int J Obes 36, 627–637 (2012). https://doi.org/10.1038/ijo.2011.213
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.213
Keywords
This article is cited by
-
Disentangling the role of NAc D1 and D2 cells in hedonic eating
Molecular Psychiatry (2023)
-
Neuroimaging Investigations of Obesity: a Review of the Treatment of Sex from 2010
Current Obesity Reports (2023)
-
Maternal pre-pregnancy BMI associates with neonate local and distal functional connectivity of the left superior frontal gyrus
Scientific Reports (2021)
-
Eating in the Absence of Hunger and Obesity Among Adolescents in Santiago, Chile
Journal of Community Health (2019)
-
Brain-Based Etiology of Weight Regulation
Current Diabetes Reports (2015)