Abstract
Background:
The majority of research on obesity (OB) has focused primarily on clinical features (eating behavior, adiposity measures) or peripheral appetite-regulatory peptides (leptin, ghrelin). However, recent functional neuroimaging studies have demonstrated that some reward circuitry regions that are associated with appetite-regulatory hormones are also involved in the development and maintenance of OB. Prader–Willi syndrome (PWS), characterized by hyperphagia and hyperghrelinemia reflecting multi-system dysfunction in inhibitory and satiety mechanisms, serves as an extreme model of genetic OB. Simple (non-PWS) OB represents an OB-control state.
Objective:
This study investigated subcortical food motivation circuitry and prefrontal inhibitory circuitry functioning in response to food stimuli before and after eating in individuals with PWS compared with OB. We hypothesized that groups would differ in limbic regions (that is, hypothalamus, amygdala) and prefrontal regions associated with cognitive control (that is, dorsolateral prefrontal cortex (DLPFC), orbitofrontal cortex (OFC) after eating.
Design and participants:
A total of 14 individuals with PWS, 14 BMI- and age-matched individuals with OB, and 15 age-matched healthy-weight controls viewed food and non-food images while undergoing functional MRI before (pre-meal) and after (post-meal) eating. Using SPM8, group contrasts were tested for hypothesized regions: hypothalamus, nucleus accumbens (NAc), amygdala, hippocampus, OFC, medial PFC and DLPFC.
Results:
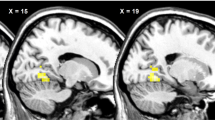
Compared with OB and HWC, PWS demonstrated higher activity in reward/limbic regions (NAc, amygdala) and lower activity in the hypothalamus and hippocampus in response to food (vs non-food) images pre-meal. Post meal, PWS exhibited higher subcortical activation (hypothalamus, amygdala, hippocampus) compared with OB and HWC. OB showed significantly higher activity versus PWS and HWC in cortical regions (DLPFC, OFC) associated with inhibitory control.
Conclusion:
In PWS, compared with OB per se, results suggest hyperactivations in subcortical reward circuitry and hypoactivations in cortical inhibitory regions after eating, which provides evidence of neural substrates associated with variable abnormal food motivation phenotypes in PWS and simple OB.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Stoeckel LE, Weller RE, Cook 3rd EW, Twieg DB, Knowlton RC, Cox JE . Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008; 41: 636–647.
Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 2007; 37: 410–421.
Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity 2010; 18: 254–260.
Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 1999; 48: 1801–1806.
Stice E, Yokum S, Burger KS, Epstein LH, Small DM . Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosc 2011; 31: 4360–4366.
Stice E, Yokum S, Blum K, Bohon C . Weight gain is associated with reduced striatal response to palatable food. J Neurosci 2010; 30: 13105–13109.
Stice E, Yokum S, Bohon C, Marti N, Smolen A . Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage 2010; 50: 1618–1625.
Alonso-Alonso M, Pascual-Leone A . The right brain hypothesis for obesity. JAMA 2007; 297: 1819–1822.
Breslin FJ, Lepping RJ, Hay TM, Martin LE, Bruce AS, Lynch AM et al. Differential encoding of food pictures in successful and unsuccessful dieters. Obesity 2010; 18 (Suppl 2): S77 (abstract 130-P).
Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes 2010; 34: 1494–1500.
Killgore WD, Yurgelun-Todd DA . Body mass predicts orbitofrontal activity during visual presentations of high-calorie foods. Neuroreport 2005; 16: 859–863.
Sescousse G, Redoute J, Dreher JC . The architecture of reward value coding in the human orbitofrontal cortex. J Neurosci 2010; 30: 13095–13104.
Hare TA, Camerer CF, Rangel A . Self-control in decision-making involves modulation of the vmPFC valuation system. Science 2009; 324: 646–648.
Burger KS, Stice E . Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipated intake, and food pictures. Neuroimage 2011; 55: 233–239.
Davids S, Lauffer H, Thoms K, Jagdhuhn M, Hirschfeld H, Domin M et al. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int J Obes 2010; 34: 94–104.
Butler MG . Prader-Willi syndrome: current understanding of cause and diagnosis. Am J Med Genet 1990; 35: 319–332.
Whittington JE, Holland AJ, Webb T, Butler J, Clarke D, Boer H . Population prevalence and estimated birth incidence and mortality rate for people with Prader-Willi syndrome in one UK Health Region. J Med Genet 2001; 38: 792–798.
Bittel DC, Butler MG . Prader-Willi syndrome: clinical genetics, cytogenetics and molecular biology. Expert Rev Mol Med 2005; 7: 1–20.
Stevenson DA, Heinemann J, Angulo M, Butler MG, Loker J, Rupe N et al. Gastric rupture and necrosis in Prader-Willi syndrome. J Pediatr Gastroenterol Nutr 2007; 45: 272–274.
Kennedy L, Bittel DC, Kibiryeva N, Kalra SP, Torto R, Butler MG . Circulating adiponectin levels, body composition and obesity-related variables in Prader-Willi syndrome: comparison with obese subjects. Int J Obes 2006; 30: 382–387.
Theodoro MF, Talebizadeh Z, Butler MG . Body composition and fatness patterns in Prader-Willi syndrome: comparison with simple obesity. Obesity 2006; 14: 1685–1690.
Butler MG, Theodoro MF, Bittel DC, Donnelly JE . Energy expenditure and physical activity in Prader-Willi syndrome: comparison with obese subjects. Am J Med Genet A 2007; 143: 449–459.
Proto C, Romualdi D, Cento RM, Romano C, Campagna G, Lanzone A . Free and total leptin serum levels and soluble leptin receptors levels in two models of genetic obesity: the Prader-Willi and the Down syndromes. Metab Clin Exp 2007; 56: 1076–1080.
Haqq AM, Farooqi IS, O′Rahilly S, Stadler DD, Rosenfeld RG, Pratt KL et al. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. J Clin Endocrinol Metab 2003; 88: 174–178.
Zipf WB, Berntson GG . Characteristics of abnormal food-intake patterns in children with Prader-Willi syndrome and study of effects of naloxone. Am J Clin Nutr 1987; 46: 277–281.
Lindgren AC, Barkeling B, Hagg A, Ritzen EM, Marcus C, Rossner S . Eating behavior in Prader-Willi syndrome, normal weight, and obese control groups. J Pediatr 2000; 137: 50–55.
Dykens EM, Maxwell MA, Pantino E, Kossler R, Roof E . Assessment of hyperphagia in Prader-Willi syndrome. Obesity 2007; 15: 1816–1826.
Dimitropoulos A, Schultz RT . Food-related neural circuitry in Prader-Willi syndrome: response to high- versus low-calorie foods. J Autism Dev Disord 2008; 38: 1642–1653.
Holsen LM, Zarcone JR, Brooks WM, Butler MG, Thompson TI, Ahluwalia JS et al. Neural mechanisms underlying hyperphagia in Prader-Willi syndrome. Obesity 2006; 14: 1028–1037.
Shapira NA, Lessig MC, He AG, James GA, Driscoll DJ, Liu Y . Satiety dysfunction in Prader-Willi syndrome demonstrated by fMRI. J Neurol Neurosurg Psychiatry 2005; 76: 260–262.
Miller JL, James GA, Goldstone AP, Couch JA, He G, Driscoll DJ et al. Enhanced activation of reward mediating prefrontal regions in response to food stimuli in Prader-Willi syndrome. J Neurol Neurosurg Psychiatry 2007; 78: 615–619.
Hinton EC, Holland AJ, Gellatly MS, Soni S, Patterson M, Ghatei MA et al. Neural representations of hunger and satiety in Prader-Willi syndrome. Int J Obes 2006; 30: 313–321.
Butler MG, Bittel DC, Kibiryeva N, Talebizadeh Z, Thompson T . Behavioral differences among subjects with Prader-Willi syndrome and type I or type II deletion and maternal disomy. Pediatrics 2004; 113: 565–573.
Stunkard AJ, Messick S . The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985; 29: 71–83.
Gelman N, Gorell JM, Barker PB, Savage RM, Spickler EM, Windham JP et al. MR imaging of human brain at 3.0 T: preliminary report on transverse relaxation rates and relation to estimated iron content. Radiology 1999; 210: 759–767.
LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM . Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci 2001; 115: 493–500.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH . An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19: 1233–1239.
Whitfield-Gabrieli S . Region of Interest Extraction (REX) Toolbox. MIT Department of Brain and Cognitive Sciences: Boston, MA, 2009.
Holsen LM, Zarcone JR, Chambers R, Butler MG, Bittel DC, Brooks WM et al. Genetic subtype differences in neural circuitry of food motivation in Prader-Willi syndrome. Int J Obes 2009; 33: 273–283.
Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS et al. Neural mechanisms underlying food motivation in children and adolescents. Neuroimage 2005; 27: 669–676.
Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res 1997; 48: 23–29.
Hosoda H, Kojima M, Kangawa K . Biological, physiological, and pharmacological aspects of ghrelin. J Pharmacol Sci 2006; 100: 398–410.
Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 1996; 273: 974–977.
Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K et al. A role for ghrelin in the central regulation of feeding. Nature 2001; 409: 194–198.
Saper CB, Chou TC, Elmquist JK . The need to feed: homeostatic and hedonic control of eating. Neuron 2002; 36: 199–211.
Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG . Central nervous system control of food intake. Nature 2000; 404: 661–671.
Holland PC, Gallagher M . Amygdala circuitry in attentional and representational processes. Trends Cogn Sci 1999; 3: 65–73.
Petrovich GD, Setlow B, Holland PC, Gallagher M . Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci 2002; 22: 8748–8753.
Schoenbaum G, Chiba AA, Gallagher M . Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature Neurosci 1998; 1: 155–159.
Zald DH . The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev 2003; 41: 88–123.
Tracy AL, Jarrard LE, Davidson TL . The hippocampus and motivation revisited: appetite and activity. Behav Brain Res 2001; 127: 13–23.
Liddle PF, Kiehl KA, Smith AM . Event-related fMRI study of response inhibition. Hum Brain Mapp 2001; 12: 100–109.
Miller EK . The prefrontal cortex and cognitive control. Nat Rev Neurosci 2000; 1: 59–65.
Tanji J, Hoshi E . Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev 2008; 88: 37–57.
DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes 2007; 31: 440–448.
Gautier JF, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M et al. Differential brain responses to satiation in obese and lean men. Diabetes 2000; 49: 838–846.
Batterink L, Yokum S, Stice E . Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage 2010; 52: 1696–1703.
Bellgrove MA, Hester R, Garavan H . The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia 2004; 42: 1910–1916.
Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S et al. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb Cortex 2009; 19: 146–152.
Evers EA, van der Veen FM, van Deursen JA, Schmitt JA, Deutz NE, Jolles J . The effect of acute tryptophan depletion on the BOLD response during performance monitoring and response inhibition in healthy male volunteers. Psychopharmacology 2006; 187: 200–208.
Woodcock KA, Humphreys GW, Oliver C, Hansen PC . Neural correlates of task switching in paternal 15q11-q13 deletion Prader-Willi syndrome. Brain Res 2010; 1363: 128–142.
Webb T, Whittington J, Clarke D, Boer H, Butler J, Holland A . A study of the influence of different genotypes on the physical and behavioral phenotypes of children and adults ascertained clinically as having PWS. Clin Genet 2002; 62: 273–281.
Lucignani G, Panzacchi A, Bosio L, Moresco RM, Ravasi L, Coppa I et al. GABA A receptor abnormalities in Prader-Willi syndrome assessed with positron emission tomography and flumazenil. Neuroimage 2004; 22: 22–28.
Kringelbach ML . The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 2005; 6: 691–702.
Stoeckel LE, Kim J, Weller RE, Cox JE, Cook 3rd EW, Horwitz B . Effective connectivity of a reward network in obese women. Brain Res Bull 2009; 79: 388–395.
Ogura K, Fujii T, Abe N, Hosokai Y, Shinohara M, Takahashi S et al. Small gray matter volume in orbitofrontal cortex in Prader-Willi syndrome: A voxel-based MRI study. Hum Brain Mapp 2010; 32: 1059–1066.
Wang Y, Beydoun MA . The obesity epidemic in the United States–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev 2007; 29: 6–28.
Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness Jr VS et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex 2001; 11: 490–497.
Acknowledgements
This study was supported by a K12-award grant to Dr Holsen from the Office for Research on Women's Health and National Institute of Child Health and Human Development (K12 HD051959), the National Institute for Child Health and Human Development (HD041672), the Hall Family Foundation, and the Heartland Genetics and Newborn Screening Collaborative (HRSA U22MC03962-02). The Hoglund Brain Imaging Center is supported by the generosity of Forrest and Sally Hoglund. The authors are grateful to Phil Lee, Allan Schmitt, Muriel Williams and Pat Weber for technical assistance and Stacey Ward, Jean Reeves, and Jean Guadagnino for help in project coordination.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Holsen, L., Savage, C., Martin, L. et al. Importance of reward and prefrontal circuitry in hunger and satiety: Prader–Willi syndrome vs simple obesity. Int J Obes 36, 638–647 (2012). https://doi.org/10.1038/ijo.2011.204
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.204
Keywords
This article is cited by
-
Cerebellar Prediction and Feeding Behaviour
The Cerebellum (2022)
-
Obesity in Prader–Willi syndrome: physiopathological mechanisms, nutritional and pharmacological approaches
Journal of Endocrinological Investigation (2021)
-
Expression and DNA Methylation Status of the Imprinted Genes PEG10 and L3MBTL1 in the Umbilical Cord Blood and Placenta of the Offspring of Assisted Reproductive Technology
Reproductive Sciences (2021)
-
Variability and change over time of weight and BMI among adolescents and adults with Prader-Willi syndrome: a 6-month text-based observational study
Orphanet Journal of Rare Diseases (2020)
-
Child neurobiology impacts success in family-based behavioral treatment for children with obesity
International Journal of Obesity (2020)