Abstract
Objective:
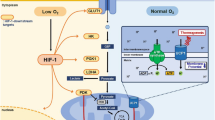
Obesity is associated with adipose tissue hypoxia, and is thought to be linked to the chronic low-grade inflammation of adipose tissue, although the precise mechanism has remained unclear. In this study, we investigated the effect of a prominent hypoxia on human primary adipocyte secretion and tumor necrosis factor alpha (TNFα)-induced nuclear factor-κB (NF-κB) signaling.
Results:
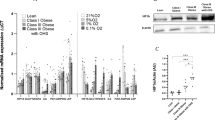
Using cytokine array and ELISA analysis, we compared the secretion patterns of normoxic and hypoxic (1% O2) adipocytes and observed various alterations in adipokine release. We could reproduce known alterations like an induction of interleukin (IL)-6, vascular endothelial growth factor, leptin and a reduction in adiponectin release under hypoxia. Interestingly, we observed a significant reduction in the secretion of macrophage chemotactic protein (MCP)-1 and other NF-κB-related genes, such as growth-regulated oncogene-α, eotaxin and soluble TNF-Receptor1 (TNF-R1) under hypoxia. TNFα stimulation of hypoxic adipocytes resulted in a significantly reduced phosphorylation of NF-κB and its inhibitor IκBα compared with normoxic cells. Furthermore, chronic treatment of hypoxic adipocytes with TNFα resulted in an expected higher secretion of the chemokines MCP-1 and IL-8, but under hypoxia, the secretion level was substantially lower than that under normoxia. This reduction in protein release was accompanied by a reduced mRNA expression of MCP-1, whereas IL-8 mRNA expression was not altered. Additionally, we observed a significantly reduced expression of the TNF-receptor TNF-R1, possibly being one cause for the reduced responsiveness of hypoxic adipocytes towards TNFα stimulation.
Conclusion:
In conclusion, human primary adipocytes show a basal and TNFα-induced reduction of MCP-1 release under hypoxia. This effect may be due to a reduced expression of TNF-R1 and therefore attenuated TNFα-induced NF-κB signaling. These observations demonstrate a reduced responsiveness of hypoxic adipocytes towards inflammatory stimuli like TNFα, which may represent an adaptation process to maintain adipose tissue function under hypoxia and inflammatory conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ahima RS . Adipose tissue as an endocrine organ. Obesity (Silver Spring) 2006; 14 (Suppl 5): 242S–249S.
Trayhurn P . Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand 2005; 184: 285–293.
Clement K, Langin D . Regulation of inflammation-related genes in human adipose tissue. J Intern Med 2007; 262: 422–430.
Wellen KE, Hotamisligil GS . Inflammation, stress and diabetes. J Clin Invest 2005; 115: 1111–1119.
Trayhurn P, Wang B, Wood IS . Hypoxia and the endocrine and signalling role of white adipose tissue. Arch Physiol Biochem 2008; 114: 267–276.
Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007; 56: 901–911.
Rausch ME, Weisberg S, Vardhana P, Tortoriello DV . Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008; 32: 451–463.
Ye J, Gao Z, Yin J, He Q . Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 2007; 293: E1118–E1128.
Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009; 58: 718–725.
Safronova O, Morita I . Transcriptome remodeling in hypoxic inflammation. J Dent Res 2010; 89: 430–444.
Dewhirst MW, Cao Y, Moeller B . Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer 2008; 8: 425–437.
Culver C, Sundqvist A, Mudie S, Melvin A, Xirodimas D, Rocha S . Mechanism of hypoxia-induced NF-kappaB. Mol Cell Biol 2010; 30: 4901–4921.
Koong AC, Chen EY, Giaccia AJ . Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res 1994; 54: 1425–1430.
Hauner H, Petruschke T, Russ M, Rohrig K, Eckel J . Effects of tumour necrosis factor alpha (TNF alpha) on glucose transport and lipid metabolism of newly-differentiated human fat cells in cell culture. Diabetologia 1995; 38: 764–771.
Dietze-Schroeder D, Sell H, Uhlig M, Koenen M, Eckel J . Autocrine action of adiponectin on human fat cells prevents the release of insulin resistance-inducing factors. Diabetes 2005; 54: 2003–2011.
Wang B, Wood IS, Trayhurn P . Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch 2007; 455: 479–492.
Wood IS, Wang B, Lorente-Cebrian S, Trayhurn P . Hypoxia increases expression of selective facilitative glucose transporters (GLUT) and 2-deoxy-D-glucose uptake in human adipocytes. Biochem Biophys Res Commun 2007; 361: 468–473.
Olefsky JM, Glass CK . Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010; 72: 219–246.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW . Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808.
Trayhurn P, Wood IS . Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004; 92: 347–355.
Murdoch C, Muthana M, Lewis CE . Hypoxia regulates macrophage functions in inflammation. J Immunol 2005; 175: 6257–6263.
Leonard EJ, Yoshimura T . Human monocyte chemoattractant protein-1 (MCP-1). Immunol Today 1990; 11: 97–101.
Sell H, Eckel J . Adipose tissue inflammation: novel insight into the role of macrophages and lymphocytes. Curr Opin Clin Nutr Metab Care 2010; 13: 366–370.
Loboda A, Stachurska A, Florczyk U, Rudnicka D, Jazwa A, Wegrzyn J et al. HIF-1 induction attenuates Nrf2-dependent IL-8 expression in human endothelial cells. Antioxid Redox Signal 2009; 11: 1501–1517.
Bosco MC, Puppo M, Pastorino S, Mi Z, Melillo G, Massazza S et al. Hypoxia selectively inhibits monocyte chemoattractant protein-1 production by macrophages. J Immunol 2004; 172: 1681–1690.
Hohensinner PJ, Kaun C, Rychli K, Ben-Tal CE, Kastl SP, Demyanets S et al. Monocyte chemoattractant protein (MCP-1) is expressed in human cardiac cells and is differentially regulated by inflammatory mediators and hypoxia. FEBS Lett 2006; 580: 3532–3538.
Negus RP, Turner L, Burke F, Balkwill FR . Hypoxia down-regulates MCP-1 expression: implications for macrophage distribution in tumors. J Leukoc Biol 1998; 63: 758–765.
Deng YY, Lu J, Ling EA, Kaur C . Monocyte chemoattractant protein-1 (MCP-1) produced via NF-kappaB signaling pathway mediates migration of amoeboid microglia in the periventricular white matter in hypoxic neonatal rats. Glia 2009; 57: 604–621.
Galindo M, Santiago B, Alcami J, Rivero M, Martin-Serrano J, Pablos JL . Hypoxia induces expression of the chemokines monocyte chemoattractant protein-1 (MCP-1) and IL-8 in human dermal fibroblasts. Clin Exp Immunol 2001; 123: 36–41.
Safronova O, Pluemsampant S, Nakahama K, Morita I . Regulation of chemokine gene expression by hypoxia via cooperative activation of NF-kappaB and histone deacetylase. Int J Biochem Cell Biol 2009; 41: 2270–2280.
Wheaton WW, Chandel NS . Hypoxia regulates Cell Metabolism. Am J Physiol Cell Physiol 2010; 300: C385–C393.
Koritzinsky M, Magagnin MG, van den Beucken T, Seigneuric R, Savelkouls K, Dostie J et al. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J 2006; 25: 1114–1125.
Oliver KM, Garvey JF, Ng CT, Veale DJ, Fearon U, Cummins EP et al. Hypoxia activates NF-kappaB-dependent gene expression through the canonical signaling pathway. Antioxid Redox Signal 2009; 11: 2057–2064.
Israel A . The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol 2010; 2: a000158.
Li H, Lin X . Positive and negative signaling components involved in TNFalpha-induced NF-kappaB activation. Cytokine 2008; 41: 1–8.
Wang M, Markel T, Crisostomo P, Herring C, Meldrum KK, Lillemoe KD et al. Deficiency of TNFR1 protects myocardium through SOCS3 and IL-6 but not p38 MAPK or IL-1beta. Am J Physiol Heart Circ Physiol 2007; 292: H1694–H1699.
Meldrum DR . Tumor necrosis factor in the heart. Am J Physiol 1998; 274: R577–R595.
Luo D, Luo Y, He Y, Zhang H, Zhang R, Li X et al. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. Am J Pathol 2006; 169: 1886–1898.
Higuchi Y, McTiernan CF, Frye CB, McGowan BS, Chan TO, Feldman AM . Tumor necrosis factor receptors 1 and 2 differentially regulate survival, cardiac dysfunction, and remodeling in transgenic mice with tumor necrosis factor-alpha-induced cardiomyopathy. Circulation 2004; 109: 1892–1897.
Shen Y, Li R, Shiosaki K . Inhibition of p75 tumor necrosis factor receptor by antisense oligonucleotides increases hypoxic injury and beta-amyloid toxicity in human neuronal cell line. J Biol Chem 1997; 272: 3550–3553.
Veroni C, Gabriele L, Canini I, Castiello L, Coccia E, Remoli ME et al. Activation of TNF receptor 2 in microglia promotes induction of anti-inflammatory pathways. Mol Cell Neurosci 2010; 45: 234–244.
Turner L, Scotton C, Negus R, Balkwill F . Hypoxia inhibits macrophage migration. Eur J Immunol 1999; 29: 2280–2287.
Sluimer JC, Daemen MJ . Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol 2009; 218: 7–29.
Acknowledgements
This work was supported by the Ministerium für Wissenschaft und Forschung des Landes Nordrhein-Westfalen, the Bundesministerium für Gesundheit, the Deutsche Forschungsgemeinschaft (SE 1922/2-1), the Commission of the European Communities (Collaborative Project ADAPT, contract number HEALTH-F2-2008–201100) and EU COST Action BM0602. We thank Prof Liebau and her team, Department of Plastic Surgery, Florence-Nightingale-Hospital Düsseldorf, and PD Dr Andree and his team, Department of Plastic Surgery and Breast Reconstruction, Sana Hospital Düsseldorf-Gerresheim, for support in obtaining adipose tissue samples. The secretarial assistance of Birgit Hurow is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Famulla, S., Horrighs, A., Cramer, A. et al. Hypoxia reduces the response of human adipocytes towards TNFα resulting in reduced NF-κB signaling and MCP-1 secretion. Int J Obes 36, 986–992 (2012). https://doi.org/10.1038/ijo.2011.200
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.200
Keywords
This article is cited by
-
Pathological roles of bone marrow adipocyte-derived monocyte chemotactic protein-1 in type 2 diabetic mice
Cell Death Discovery (2023)
-
Cytokines and inflammation in adipogenesis: an updated review
Frontiers of Medicine (2019)
-
Free fatty acid G-protein coupled receptor signaling in M1 skewed white adipose tissue macrophages
Cellular and Molecular Life Sciences (2016)