Abstract
Objective:
To investigate whether the paradoxical weight gain associated with dieting is better related to genetic propensity to weight gain than to the weight loss episodes themselves.
Subjects:
Subjects included 4129 individual twins from the population-based FinnTwin16 study (90% of twins born in Finland 1975–1979). Weight and height were obtained from longitudinal surveys at 16, 17, 18 and 25 years, and number of lifetime intentional weight loss (IWL) episodes of more than 5 kg at 25 years.
Results:
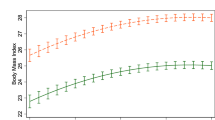
IWLs predicted accelerated weight gain and risk of overweight. The odds of becoming overweight (body mass index (BMI)⩾25 kg m−2) by 25 years were significantly greater in subjects with one (OR 1.8, 95% CI 1.3–2.6, and OR 2.7, 1.7–4.3 in males and females, respectively), or two or more (OR 2.0, 1.3–3.3, and OR 5.2, 3.2–8.6, in males and females, respectively), IWLs compared with subjects with no IWL. In MZ pairs discordant for IWL, co-twins with at least one IWL were 0.4 kg m−2 (P=0.041) heavier at 25 years than their non-dieting co-twins (no differences in baseline BMIs). In DZ pairs, co-twins with IWLs gained progressively more weight than non-dieting co-twins (BMI difference 1.7 kg m−2 at 16 years and 2.2 kg m−2 at 25 years, P<0.001).
Conclusion:
Our results suggest that frequent IWLs reflect susceptibility to weight gain, rendering dieters prone to future weight gain. The results from the MZ pairs discordant for IWLs suggest that dieting itself may induce a small subsequent weight gain, independent of genetic factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cannon G, Einzig H . Dieting Makes You Fat. Century Publishing: London, 1983.
Mann T, Tomiyama AJ, Westling E, Lew AM, Samuels B, Chatman J . Medicare's search for effective obesity treatments: diets are not the answer. Am Psychol 2007; 62: 220–233.
Hill AJ . Does dieting make you fat? Br J Nutr 2004; 92 (Suppl 1): S15–S18.
French SA, Jeffery RW, Forster JL, McGovern PG, Kelder SH, Baxter JE . Predictors of weight change over two years among a population of working adults: the Healthy Worker Project. Int J Obes 1994; 18: 145–154.
Bild DE, Sholinsky P, Smith DE, Lewis CE, Hardin JM, Burke GL . Correlates and predictors of weight loss in young adults: the CARDIA study. Int J Obes 1996; 20: 47–55.
Coakley EH, Rimm EB, Colditz G, Kawachi I, Willett W . Predictors of weight change in men: results from the Health Professionals Follow-up Study. Int J Obes 1998; 22: 89–96.
Kroke A, Liese AD, Schulz M, Bergmann MM, Klipstein-Grobusch K, Hoffmann K et al. Recent weight changes and weight cycling as predictors of subsequent two year weight change in a middle-aged cohort. Int J Obes 2002; 26: 403–409.
Field AE, Aneja P, Austin SB, Shrier LA, de MC, Gordon-Larsen P . Race and gender differences in the association of dieting and gains in BMI among young adults. Obesity 2007; 15: 456–464.
Korkeila M, Rissanen A, Kaprio J, Sorensen TI, Koskenvuo M . Weight-loss attempts and risk of major weight gain: a prospective study in Finnish adults. Am J Clin Nutr 1999; 70: 965–975.
Stice E, Cameron RP, Killen JD, Hayward C, Taylor CB . Naturalistic weight-reduction efforts prospectively predict growth in relative weight and onset of obesity among female adolescents. J Consult Clin Psychol 1999; 67: 967–974.
Neumark-Sztainer D, Wall M, Haines J, Story M, Eisenberg ME . Why does dieting predict weight gain in adolescents? Findings from project EAT-II: a 5-year longitudinal study. J Am Diet Assoc 2007; 107: 448–455.
Polivy J, Herman CP . Dieting and binging. A causal analysis. Am Psychol 1985; 40: 193–201.
Prentice AM, Goldberg GR, Jebb SA, Black AE, Murgatroyd PR, Diaz EO . Physiological responses to slimming. Proc Nutr Soc 1991; 50: 441–458.
Dulloo AG, Jacquet J, Montani JP . Pathways from weight fluctuations to metabolic diseases: focus on maladaptive thermogenesis during catch-up fat. Int J Obes 2002; 26 (Suppl 2): S46–S57.
Keski-Rahkonen A, Neale BM, Bulik CM, Pietiläinen KH, Rose RJ, Kaprio J et al. Intentional weight loss in young adults: sex-specific genetic and environmental effects. Obes Res 2005; 13/4: 745–753.
Kaprio J, Pulkkinen L, Rose RJ . Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Res 2002; 5: 366–371.
Saarni SE, Pietiläinen K, Kantonen S, Rissanen A, Kaprio J . Association of smoking in adolescence with abdominal obesity in adulthood: a follow-up study of 5 birth cohorts of Finnish twins. Am J Public Health 2009; 99: 348–354.
Sarna S, Kaprio J, Sistonen P, Koskenvuo M . Diagnosis of twin zygosity by mailed questionnaire. Hum Hered 1978; 28: 241–254.
Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R et al. CDC growth charts: United States. Adv Data 2000; 314: 1–27.
Cole TJ, Green PJ . Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 1992; 11: 1305–1319.
Wehkalampi K, Silventoinen K, Kaprio J, Dick DM, Rose RJ, Pulkkinen L et al. Genetic and environmental influences on pubertal timing assessed by height growth. Am J Hum Biol 2008; 20: 417–423.
Aarnio M, Winter T, Kujala U, Kaprio J . Associations of health related behaviour, social relationships, and health status with persistent physical activity and inactivity: a study of Finnish adolescent twins. Br J Sports Med 2002; 36: 360–364.
Keski-Rahkonen A, Bulik CM, Pietiläinen KH, Rose RJ, Kaprio J, Rissanen A . Eating styles, overweight and obesity in young adult twins. Eur J Clin Nutr 2007; 61: 822–829.
Classification of Socio-Economic Groups. Central Statistical Office of Finland: Helsinki, Finland, 1989.
Rao JNK, Scott AJ . On chi-squared tests for multiway contingency tables with cell proportions estimated from survey data. Ann Stat 1984; 12: 46–60.
Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL . The Biology of Human Starvation. University of Minnesota Press: Minnesota, USA, 1950.
Womble LG, Williamson DA, Martin CK, Zucker NL, Thaw JM, Netemeyer R et al. Psychosocial variables associated with binge eating in obese males and females. Int J Eat Disord 2001; 30: 217–221.
Swanson DW, Dinello FA . Follow-up of patients starved for obesity. Psychosom Med 1970; 32: 209–214.
Ernsberger P, Koletsky RJ, Baskin JS, Collins LA . Consequences of weight cycling in obese spontaneously hypertensive rats. Am J Physiol 1996; 270/4 (Part 2): R864–R872.
Kajioka T, Tsuzuku S, Shimokata H, Sato Y . Effects of intentional weight cycling on non-obese young women. Metabolism 2002; 51: 149–154.
Dulloo AG, Jacquet J, Girardier L . Autoregulation of body composition during weight recovery in human: the Minnesota Experiment revisited. Int J Obes 1996; 20: 393–405.
Wadden TA, Foster GD, Stunkard AJ, Conill AM . Effects of weight cycling on the resting energy expenditure and body composition of obese women. Int J Eat Disord 1996; 19: 5–12.
Jebb SA, Goldberg GR, Coward WA, Murgatroyd PR, Prentice AM . Effects of weight cycling caused by intermittent dieting on metabolic rate and body composition in obese women. Int J Obes 1991; 15: 367–374.
Forbes GB . Body fat content influences the body composition response to nutrition and exercise. Ann NY Acad Sci 2000; 904: 359–365.
Weinsier RL, Hunter GR, Zuckerman PA, Redden DT, Darnell BE, Larson DE et al. Energy expenditure and free-living physical activity in Black and White women: comparison before and after weight loss. Am J Clin Nutr 2000; 71: 1138–1146.
Rosenbaum M, Hirsch J, Murphy E, Leibel RL . Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr 2000; 71: 1421–1432.
Loos RJ, Rankinen T . Gene–diet interactions on body weight changes. J Am Diet Assoc 2005; 105 (Suppl 1): S29–S34.
Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G et al. The response to long-term overfeeding in identical twins. N Engl J Med 1990; 322: 1477–1482.
Bouchard C, Tremblay A, Despres JP, Theriault G, Nadeau A, Lupien PJ et al. The response to exercise with constant energy intake in identical twins. Obes Res 1994; 2: 400–410.
Saarni SE, Rissanen A, Sarna S, Koskenvuo M, Kaprio J . Weight cycling of athletes and subsequent weight gain in middleage. Int J Obes 2006; 30: 1639–1644.
Field AE, Haines J, Rosner B, Willett WC . Weight-control behaviors and subsequent weight change among adolescents and young adult females. Am J Clin Nutr 2010; 91: 147–153.
Li S, Zhao JH, Luan J, Ekelund U, Luben RN, Khaw KT et al. Physical activity attenuates the genetic predisposition to obesity in 20 000 men and women from EPIC-Norfolk prospective population study. PLoS Med 2010; 7: e1000332.
Acknowledgements
We thank the FinnTwin16 participants for their invaluable help in this prospective study. The study was supported by Helsinki University Hospital Research Funds (AR, KHP), grants from Novo Nordisk, Gyllenberg, Yrjö Jahnsson, Biomedicum Helsinki, the Jalmari and Rauha Ahokas Foundation, and the Finnish Foundation for Cardiovascular Research (KHP), the Väinö and Laina Kivi Foundation (SES), the Academy of Finland Centre of Excellence in Complex Disease Genetics (Grants 213506 and 129680 to JK). Data collection in FinnTwin16 was supported by the National Institute of Alcohol Abuse and Alcoholism (Grants AA-12502 and AA-09203 to Richard J Rose), and the Academy of Finland (Grants 44069 and 141054 to JK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pietiläinen, K., Saarni, S., Kaprio, J. et al. Does dieting make you fat? A twin study. Int J Obes 36, 456–464 (2012). https://doi.org/10.1038/ijo.2011.160
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.160
Keywords
This article is cited by
-
Weight Bias in Obstetrics
Current Obstetrics and Gynecology Reports (2023)
-
Mindful Eating as a Tool for Diabetes Prevention and Management: A Review of Potential Mechanisms of Action
Mindfulness (2023)
-
Associations of body mass index, fasting insulin, and inflammation with mortality: a prospective cohort study
International Journal of Obesity (2022)
-
Associations between weight loss history and factors related to type 2 diabetes risk in the Stop Diabetes study
International Journal of Obesity (2022)
-
Clinical Considerations of Ultra-processed Food Addiction Across Weight Classes: an Eating Disorder Treatment and Care Perspective
Current Addiction Reports (2022)