Abstract
Objective:
In this study, we investigate the brain mechanisms of the conscious regulation of the desire for food using functional magnetic resonance imaging. Further, we examine associations between hemodynamic responses and participants’ cognitive restraint of eating (CRE), as well as their susceptibility to uncontrolled eating.
Subjects:
Seventeen non-vegetarian, right-handed, female Caucasian participants (age: 20–30 years, mean 25.3 years±3.1 s.d.; BMI: 20.2–31.2 kg m−2, mean 25.1±3.5 s.d.).
Measurements:
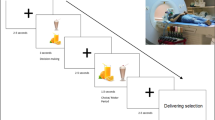
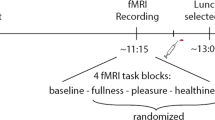
During scanning, our participants viewed pictures of food items they had pre-rated according to tastiness and healthiness. Participants were either allowed to admit to the desire for the food (ADMIT) or they were instructed to downregulate their desire using a cognitive reappraisal strategy, that is, thinking of negative long-term health-related and social consequences (REGULATE).
Results:
Comparing the hemodynamic responses of the REGULATE with the ADMIT condition, we observed robust activation in the dorsolateral prefrontal cortex (DLPFC), the pre-supplementary motor area, the inferior frontal gyrus (IFG), the dorsal striatum (DS), the bilateral orbitofrontal cortex (OFC), the anterior insula and the temporo-parietal junction (TPJ). Activation in the DLPFC and the DS strongly correlated with the degree of dietary restraint under both conditions.
Conclusion:
Cortical activation in the DLPFC, the pre-supplementary motor area and the inferior frontal gyrus (IFG) are known to underpin top-down control, inhibition of learned associations and pre-potent responses. The observed hemodynamic responses in the lateral OFC, the DS, the anterior insula and the TPJ support the notion of reward valuation and integration, interoceptive awareness, and self-reflection as key processes during active regulation of desire for food. In conclusion, an active reappraisal of unhealthy food recruits the brain's valuation system in combination with prefrontal cognitive control areas associated with response inhibition. The correlations between brain responses and CRE suggest that individuals with increased cognitive restraint show an automatic predisposition to regulate the hedonic aspects of food stimuli. This cognitive control might be necessary to counterbalance a lack of homeostatic mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 2010; 363: 2211–2219.
Withrow D, Alter DA . The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev 2011; 12: 131–141.
Van Vugt DA . Brain imaging studies of appetite in the context of obesity and the menstrual cycle. Hum Reprod Update 2010; 16: 276–292.
Kringelbach ML . Food for thought: hedonic experience beyond homeostasis in the human brain. Neuroscience 2004; 126: 807–819.
Stice E, Yokum S, Blum K, Bohon C . Weight gain is associated with reduced striatal response to palatable food. J Neurosci 2010; 30: 13105–13109.
Zald DH . Orbitofrontal cortex contributions to food selection and decision making. Ann Behav Med 2009; 38: 18–24.
Kringelbach ML, Radcliffe J . The human orbitofrontal cortex: linking reward to hedonic experience. Neuroscience 2005; 6: 691–702.
Hare TA, Camerer CF, Rangel A . Self-control in decision-making involves modulation of the vmPFC valuation system. Science 2009; 646: 646–648.
Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL et al. Prefrontal–striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci USA 2010; 107: 14811–14816.
Hellerstein MK, Benowitz NL, Neese RA, Schwartz JM, Hoh R, Jacob III P et al. Effects of cigarette smoking and its cessation on lipid metabolism and energy expenditure in heavy smokers. J Clin Invest 1994; 93: 265–272.
Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry 2008; 165: 179–187.
Ochsner KN, Gross JJ . The cognitive control of emotion. Trends Cogn Sci 2005; 9: 242–249.
Morewedge CK, Huh YE, Vosgerau J . Thought for food: imagined consumption reduces actual consumption. Science 2010; 330: 1530–1533.
Stunkard AJ, Messick S . The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985; 29: 71–83.
Pudel V, Westenhöfer J . Fragebogen zum Eßverhalten (FEV). Handanweisung: Göttingen, 1989.
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J . An inventory for measuring depression. Arch Gen Psychiat 1961; 4: 561–571.
Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci USA 2010; 107: 6106–6111.
Swick D, Ashley V, Turken AU . Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci 2008; 9: 102.
Caplan JB, McIntosh AR, De Rosa E . Two distinct functional networks for successful resolution of proactive interference. Cereb Cortex 2007; 17: 1650–1663.
Corbetta M, Shulman GL . Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002; 3: 201–215.
Levens SM, Phelps EA . Insula and orbital frontal cortex activity underlying emotion interference resolution in working memory. J Cognitive Neurosci 2010; 22: 2790–2803.
Critchley HD . Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 2005; 493: 154–166.
Suhler CL, Churchland PS . Control: conscious and otherwise. Trends Cogn Sci 2009; 13: 341–347.
Jonides J, Nee DE . Brain mechanisms of proactive interference in working memory. Neuroscience 2006; 139: 181–193.
Kahnt T, Heinzle J, Park SQ, Haynes J-D . The neural code of reward anticipation in human orbitofrontal cortex. Proc Natl Acad Sci USA 2010; 107: 6010–6015.
Kennerley SW, Dahmubed AF, Lara AH, Wallis JD . Neurons in the frontal lobe encode the value of multiple decision variables. J Cogn Neurosci 2009; 21: 1162–1178.
Peters J, Büchel C . Neural representations of subjective reward value. Behav Brain Res 2010; 213: 135–141.
Vorhold V, Giessing C, Wiedemann PM, Schütz H, Gauggel S, Fink GR . The neural basis of risk ratings: evidence from a functional magnetic resonance imaging (fMRI) study. Neuropsychologia 2007; 45: 3242–3250.
Saxe R, Moran JM, Scholz J, Gabrieli J . Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Soc Cogn Affect Neurosci 2006; 1: 229–234.
Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ . Neural systems supporting interoceptive awareness. Nat Neurosci 2004; 7: 189–195.
Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJM et al. Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci 2008; 28: 7143–7152.
Haber SN, Knutson B . The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35: 4–26.
O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ . Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 2004; 304: 452–454.
Koechlin E, Ody C, Kouneiher F . The architecture of cognitive control in the human prefrontal cortex. Science 2003; 302: 1181–1185.
Cornier M-A, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR . Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav 2010; 99: 538–543.
Rolls BJ, Fedoroff IC, Guthrie JF . Gender differences in eating behavior and body weight regulation. Health Psychol 1991; 10: 133–142.
Provencher V, Drapeau V, Tremblay A, Després J-P, Lemieux S . Eating behaviors and indexes of body composition in men and women from the Québec family study. Obes Res 2003; 11: 783–792.
Carroll JF, Kaiser KA, Franks SF, Deere C, Caffrey JL . Influence of BMI and gender on postprandial hormone responses. Obesity 2007; 15: 2974–2983.
Beasley JM, Ange BA, Anderson CAM, Miller Iii ER, Holbrook JT, Appel LJ . Characteristics associated with fasting appetite hormones (obestatin, ghrelin, and leptin). Obesity 2009; 17: 349–354.
DelParigi A, Pannacciulli N, Le DN, Tataranni PA . In pursuit of neural risk factors for weight gain in humans. Neurobiol Aging 2005; 26 (Suppl 1): 50–55.
Wang G-J, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci USA 2009; 106: 1249–1254.
Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC . Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res 2006; 169: 111–119.
Stice E, Spoor S, Bohon C, Small DM . Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 2008; 322: 449–452.
Bruce a S, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obesity 2010; 34: 1494–1500.
Stice E, Yokum S, Bohon C, Marti N, Smolen A . Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. NeuroImage 2010; 50: 1618–1625.
Acknowledgements
We are thankful to Rosie Wallis, who proofread the manuscript. This work was supported by the Max Planck Society, the Federal Ministry of Education and Research (BMBF: Neurocircuits in obesity to AH, HS, MS, AV and BP; IFB AdiposityDiseases (FKZ: 01EO1001) to AH, AV and MS) and the European Union (GIPIO to HS and MS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hollmann, M., Hellrung, L., Pleger, B. et al. Neural correlates of the volitional regulation of the desire for food. Int J Obes 36, 648–655 (2012). https://doi.org/10.1038/ijo.2011.125
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.125
Keywords
This article is cited by
-
State-specific alterations in the neural computations underlying inhibitory control in women remitted from bulimia nervosa
Molecular Psychiatry (2023)
-
Neural activation of regions involved in food reward and cognitive control in young females with anorexia nervosa and atypical anorexia nervosa versus healthy controls
Translational Psychiatry (2023)
-
Food stimuli decrease activation in regions of the prefrontal cortex related to executive function: an fNIRS study
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2023)
-
Neurobiological regulation of eating behavior: Evidence based on non-invasive brain stimulation
Reviews in Endocrine and Metabolic Disorders (2022)
-
Interactions between emotions and eating behaviors: Main issues, neuroimaging contributions, and innovative preventive or corrective strategies
Reviews in Endocrine and Metabolic Disorders (2022)