Abstract
Background:
Obesity is linked to chronic inflammation in white adipose tissue, which is exacerbated by infiltrating macrophages (MΦs). We recently demonstrated that an extract from grape powder (GPE), which is abundant in quercetin (QUE), reduced inflammation in human MΦs and prevented MΦ-mediated inflammation and insulin resistance in human adipocytes. However, we did not know how QUE individually affected these outcomes.
Objective and design:
We examined the extent to which QUE prevents inflammation in human MΦs (that is, differentiated U937 cell line) and cross-talk with human adipocytes (that is, primary cultures of newly differentiated human adipocytes).
Methods and results:
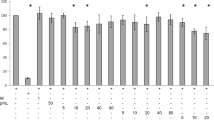
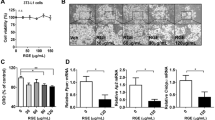
Treatment of MΦs with QUE attenuated the basal expression of inflammatory genes, such as tumor necrosis factor-α, interleukin (IL)-6, IL-8, IL-1β and interferon-γ inducible protein-10, and cyclooxygenase-2, a marker of prostaglandin production. QUE also attenuated the abundance of phosphorylated c-Jun N-terminal kinase (JNK) and c-Jun, and IκBα degradation in MΦs. Furthermore, conditioned media (CM) obtained from MΦs treated with QUE decreased the capacity of this CM to inflame adipocytes and cause insulin resistance as evidenced by decreased: (1) inflammatory gene expression, (2) phosphorylation of JNK and c-Jun, (3) serine residue 307 phosphorylation of insulin receptor substrate (IRS)-1, 4) protein tyrosine phosphatase-1B gene expression and 5) suppression of insulin-stimulated glucose uptake.
Conclusion:
Taken together, these data suggest that QUE is one of the bioactive components of GPE that prevents inflammation in MΦs and MΦ-mediated insulin resistance in adipocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Tilg H, Moschen AR . Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006; 6: 772–783.
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on obesity and heart disease from the Obesity Committee of the Council on nutrition, physical activity, and metabolism. Circulation 2006; 113: 898–918.
Cottam DR, Mattar SG, Barinas-Mitchell E, Eid G, Kuller L, Kelley DE et al. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg 2004; 14: 589–600.
Trayhurn P, Wood IS . Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004; 92: 347–355.
Laine PS, Schwartz EA, Wang Y, Zhang WY, Karnik SK, Musi N et al. Palmitic acid induces IP-10 expression in human macrophages via NF-kappaB activation. Biochem Biophys Res Commun 2007; 358: 150–155.
Wellen KE, Hotamisligil GS . Inflammation, stress, and diabetes. J Clin Invest 2005; 115: 1111–1119.
Fantuzzi G . Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 2004; 115: 911–919.
Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW . Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissue of obese humans. Endocrinology 2004; 145: 2273–2282.
Clément K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J 2004; 18: 1657–1669.
Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 2006; 49: 744–747.
Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R . MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006; 116: 1494–1505.
Skurk T, Herder C, Kraft I, Muller-Scholze S, Hauner H, Kolb H . Production and release of macrophage migration inhibitory factor from human adipocytes. Endocrinology 2005; 146: 1006–1011.
Lumeng C, Deyoung S, Saltiel A . Macrophages block insulin action in adipcytes by altering expression of signaling and glucose transport proteins. Am J Physiol 2007; 292: E166–E174.
Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005; 11: 191–198.
Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2005; 116: 115–124.
Sakurai T, Kitadateb K, Nishiokab H, Fujiib H, Kizakia T, Kondohc Y et al. Oligomerized grape seed polyphenols attenuate inflammatory changes due to antioxidative properties in coculture of adipocytes and macrophages. J Nutr Biochem 2010; 21: 47–54.
Terra X, Valls J, Vitrac X, Mérrillon JM, Arola L, Ardèvol A et al. Grape-seed procyanidins act as antiinflammatory agents in endotoxin-stimulated RAW 264.7 macrophages by inhibiting NFkB signaling pathway. J Agric Food Chem 2007; 55: 4357–4365.
Overman A, Bumrungpert A, Kennedy A, Martinez K, Chuang C, West T et al. Polyphenol-rich grape extract attenuates inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. Int J Obesity 2010; 34: 800–808.
Matheson LA, Labow RS, Santerre JP . Biodegradation of polycarbonate-based polyurethanes by the human monocytes-derived macrophage and U937 cell systems. J Biomed Mater Res 2002; 61: 505–513.
Brown JM, Sandberg-Boysen M, Chung S, Fabiyi O, Morrision R, Mandrup S et al. Conjugated linoleic acid (CLA) induces human adipocyte delipidation: autocrine/paracrine regulation of MEK/ERK signaling by adipocytokines. J Biol Chem 2004; 279: 26735–26747.
Chung S, Lapoint K, Martinez K, Kennedy A, Boysen Sandberg M, McIntosh MK . Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology 2006; 147: 5340–5351.
Yin K, Liao D, Tang C . ABAC1: a possible link between inflammation and reverse cholesterol transport. Mol Med 2010; 16: 438–449.
Suganami T, Nishida H, Ogawa Y . A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and TNFα. Arterioscler Throm Vasc Biol 2005; 25: 2062–2068.
Egert S, Bosy-Westphal A, Seiberl J, Kürbitz C, Settler U, Plachta-Danielzik S et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr 2009; 102: 1065–1074.
Stewart LK, Soileau JL, Ribnicky D, Wang ZQ, Raskin I, Poulev A et al. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metabolism 2008; 57: S39–S46.
Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M . Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity 2008; 16: 2081–2087.
Loke WM, Proudfoot JM, Hodgson JM, McKinley AJ, Hime N, Magat M et al. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler Thromb Vasc Biol 2010; 30: 749–757.
Okoko T, Oruambo IF . Inhibitory activity of quercetin and its metabolite on lipopolysaccharide-induced activation of macrophage U937 cells. Food Chem Toxico 2009; 47: 809–812.
Kaneko M, Takimoto H, Sugiyama T, Seki Y, Kawaguchi K, Kumazawa Y . Suppressive effects of the flavonoids quercetin and luteolin on the accumulation of lipid rafts after signal transduction via receptors. Immunopharmacol Immunotoxicol 2008; 30: 867–882.
Ahn J, Lee H, Kim S, Park J, Ha T . The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun 2008; 373: 545–549.
Lee KW, Kang NJ, Heo YS, Rogozin EA, Pugliese A, Hwang MK et al. Raf and MEK protein kinases are direct molecular targets for the chemopreventive effect of quercetin, a major flavonol in red wine. Cancer Res 2008; 68: 946–955.
Huang RY, Yu YL, Cheng WC, Ouyang CN, Fu E, Chu CL . Immunosuppressive effect of quercetin on dendritic cell activation and function. J Immunol 2010; 184: 6815–6821.
Ortega MG, Saragusti AC, Cabrera JL, Chiabrando GA . Quercetin tetraacetyl derivative inhibits LPS-induced nitric oxide synthase (iNOS) expression in J774A.1 cells. Arch Biochem Biophys 2010; 498: 105–110.
Jang S, Kelley KW, Johnson RW . Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci USA 2008; 105: 7534–7539.
Chen CY, Peng WH, Tsai KD, Hsu SL . Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci 2007; 81: 1602–1614.
Goldstein BJ, Bittner-Kowalczyk A, White MF, Harbeck M . Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb.2adaptor protein. J Biol Chem 2000; 275: 4283–4289.
Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB . Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem 2008; 283: 14230–14241.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112: 1821–1830.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW . Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808.
Permana PA, Menge C, Reaven PD . Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem Biophys Res Commun 2006; 341: 507–514.
Rahman I, Biswas S, Kirkham P . Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharm 2006; 72: 1439–1452.
Necela BM, Su W, Thompson EA . Toll-like receptor 4 mediates cross-talk between peroxisome proliferator-activated receptor γ and nuclear factor-κB in macrophages. Immunology 2008; 125: 344–358.
Su CG, Wen X, Bailey ST, Jiang W, Rangwala SM, Keilbaugh SA et al. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J Clin Invest 1999; 104: 383–389.
Desreumaux P, Dubuquoy L, Nutten S, Peuchmaur M, Englaro W, Schoonjans K et al. Attenuation of colon inflammation through activators of the retinoid X receptor(RXR)/peroxisome proliferator-activated receptor gamma(PPARgamma) heterodimer. A basis for new therapeutic strategies. J Exp Med 2001; 193: 827–838.
Saubermann LJ, Nakajima A, Wada K, Zhao S, Terauchi Y, Kadowaki T et al. Peroxisome proliferator-activated receptor gamma agonist ligands stimulate a Th2 cytokine response and prevent acute colitis. Inflamm Bowel Dis 2002; 8: 330–339.
Katayama K, Wada K, Nakajima A, Mizuguchi H, Hayakawa T, Nakagawa S et al. A novel PPAR gamma gene therapy to control inflammation associated with inflammatory bowel disease in a murine model. Gastroenterology 2003; 124: 1315–1324.
Lytle C, Tod TJ, Vo KT, Lee JW, Atkinson RD, Straus DS . The peroxisome proliferator-activated receptor gamma ligand rosiglitazone delays the onset of inflammatory bowel disease in mice with interleukin 10 deficiency. Inflamm Bowel Dis 2005; 11: 231–243.
Schaefer KL, Denevich S, Ma C, Cooley SR, Nakajima A, Wada K et al. Intestinal antiinflammatory effects of thiazolidenedione peroxisome proliferator-activated receptor-gamma ligands on T helper type 1 chemokine regulation include nontranscriptional control mechanisms. Inflamm Bowel Dis 2005; 11: 244–252.
Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM . Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 1998; 93: 229–240.
Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM . PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 1998; 93: 241–252.
Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK . Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest 2000; 106: 523–531.
Zhang L, Chawla A . Role of PPARgamma in macrophage biology and atherosclerosis. Trends Endocrinol Metab 2004; 15: 500–505.
Hammad H, de Heer HJ, Soullie T, Angeli V, Trottein F, Hoogsteden HC et al. Activation of peroxisome proliferator activated receptor-gamma in dendritic cells inhibits the development of eosinophilic airway inflammation in a mouse model of asthma. Am J Pathol 2004; 164: 263–271.
Woerly G, Honda K, Loyens M, Papin JP, Auwerx J, Staels B et al. Peroxisome proliferator-activated receptors alpha and gamma down-regulate allergic inflammation and eosinophil activation. J Exp Med 2003; 198: 411–421.
Trifilieff A, Bench A, Hanley M, Bayley D, Campbell E, Whittaker P . PPAR-alpha and -gamma but not -delta agonists inhibit airway inflammation in a murine model of asthma: in vitro evidence for an NF-kappaB-independent effect. Br J Pharmacol 2003; 139: 163–171.
Demerjian M, Man MQ, Choi EH, Brown BE, Crumrine D, Chang S et al. Topical treatment with thiazolidinediones, activators of peroxisome proliferator-activated receptor-gamma, normalizes epidermal homeostasis in a murine hyperproliferative disease model. Exp Dermatol 2006; 15: 154–160.
Hasegawa H, Takano H, Zou Y, Qin Y, Hizukuri K, Odaka K et al. Pioglitazone, a peroxisome proliferator activated receptor gamma activator, ameliorates experimental autoimmune myocarditis by modulating Th1/Th2 balance. J Mol Cell Cardiol 2005; 38: 257–265.
Yuan ZY, Liu Y, Liu Y, Zhang JJ, Kishimoto C, Wang YN et al. PPAR-gamma ligands inhibit the expression of inflammatory cytokines and attenuate autoimmune myocarditis. Chin Med J (Engl) 2004; 117: 1253–1255.
Natarajan C, Bright JJ . Peroxisome proliferator-activated receptor-gamma agonists inhibit experimental allergic encephalomyelitis by blocking IL-12 production, IL-12 signaling and Th1differentiation. Genes Immun 2002; 3: 59–70.
Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE et al. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandinJ(2) ameliorates experimental autoimmune encephalomyelitis. J Immunol 2002; 168: 2508–2515.
Wang Q, Xia M, Liu C, Guo H, Ye Q, Hu Y et al. Cyanidin-3-O-beta-glucoside inhibits iNOS and COX-2 expression by inducing liver X receptor alpha activation in THP-1 macrophages. Life Sci 2008; 83: 176–184.
Xia M, Hou M, Zhu H, Ma J, Tang Z, Wang Q et al. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: the role of the peroxisome proliferator-activated receptor γ-liver X receptor α-ABCA1 pathway. J Biol Chem 2005; 280: 36792–36801.
Szanto A, Rõszer T . Nuclear receptors in macrophages: a link between metabolism and inflammation. FEBS Letters 2008; 582: 106–116.
Varga T, Nagy L . Nuclear receptors, transcription factors linking lipid metabolism and immunity: the case of peroxisome proliferator-activated receptor gamma. Eur J Clin Invest 2008; 38: 695–707.
Acknowledgements
We thank the North Carolina Agricultural Research Service (NCARS 02288) for providing financial support for these studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Overman, A., Chuang, CC. & McIntosh, M. Quercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned media. Int J Obes 35, 1165–1172 (2011). https://doi.org/10.1038/ijo.2010.272
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.272
Keywords
This article is cited by
-
The multifaceted role of quercetin derived from its mitochondrial mechanism
Molecular and Cellular Biochemistry (2023)
-
No effects of quercetin from onion skin extract on serum leptin and adiponectin concentrations in overweight-to-obese patients with (pre-)hypertension: a randomized double-blinded, placebo-controlled crossover trial
European Journal of Nutrition (2017)
-
Quercetin reduces obesity-induced hepatosteatosis by enhancing mitochondrial oxidative metabolism via heme oxygenase-1
Nutrition & Metabolism (2015)
-
Acerola (Malpighia emarginata DC.) juice intake protects against alterations to proteins involved in inflammatory and lipolysis pathways in the adipose tissue of obese mice fed a cafeteria diet
Lipids in Health and Disease (2014)